2940
Assessment of age-related changes of oxygen extraction fraction in of normal adults using QSM and quantitative BOLD1the Fourth Affiliated Hospital of China Medical University, Shenyang, China, 2Weill Cornell Medicine, New York, NY, United States, 3MR Scientific Marketing, Siemens Healthineers, Beijing, China, 4Shengjing Hospital of China Medical University, Shenyang, China
Synopsis
With aging, the brain undergoes comprehensive changes in its function and physiology, including oxygen consumption metabolism. In this study of healthy aging from 24-69, oxygen extraction fraction (OEF) mapping was generated using QSM and quantitative BOLD (QSM+qBOLD, QQ) processing of challenge-free 3D multi-echo gradient echo (mGRE). OEF values were found to increase with age only in specific gray matter brain regions (mainly in SomMot and SalVentAttn functional networks) and to decrease with age only in specific white matter brain regions (mainly Callosum ,cingulate,superior frontal blade).
Introduction
With age, many aspects of the brain structure undergo a pronounced decline, yet individuals generally function well until advanced old age. Though brain region-specific oxygen metabolism is highly required in aging study, previous studies mostly focused on whole brain average oxygen extraction fraction (OEF), and the quantitative measurement of OEF after accurate division of specific brain areas has not been reported.Recently, a novel combined model of quantitative susceptibility mapping (QSM) and quantitative BOLD (QSM+qBOLD or QQ) has been developed to map OEF without vascular challenges by utilizing both magnitude and phase of multi-echo gradient echo (mGRE) data. QQ has been further validated against calibrated fMRI and 15O-PET. In the current study, we assessed the age-related changes of OEF in normal adults using QQ method.
Methods
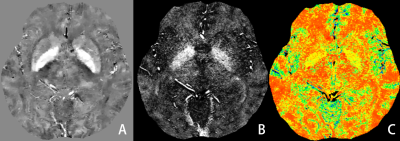
mGRE was acquired from 25 healthy subjects (men=8, ages 24-69) using 3D multi-echo GRE sequence on a 3-T MRI system. The QSM and OEF were postprocessed using QQ software package developed by Department of Radiology, Weill Cornell Medicine. QSM mapping was reconstructed using a fully automated zero-referenced morphology enabled dipole inversion method that uses the ventricular cerebrospinal fluid (CSF) as a zero reference. OEF maps were then calculated based on QSM and mGRE magnitude using the CCTV-based QQ model. The OEF maps were then normalized using FSL software package and the resultant data were segmented into 100 gray matter regions and 68 white matter regions using functional brain template described by Schaefer and HarvardOxford white template. Pearson correlation analysis was performed between the age and OEF in each brain region.Results
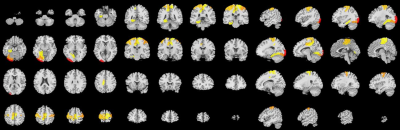
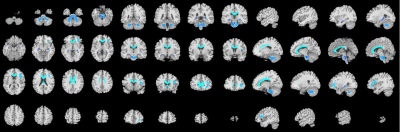
The OEF significantly increased with age in specific gray matter regions, mainly in somatomotor (SomMot) and salience/ventral attention (SalVentAttn) functional networks. However, the white matter of callosum, cingulate, superior frontal blade showed negative correlation with age.Discussion
This study demonstrated the feasibility of QQ method to study aging related cerebral OEF change. The increased OEF with age in gray matter regions such as bilateral SomMot and left SalVentAttn may be related to the function of these areas and the relatively high oxygen consumption. This may be associated with compensatory response to reduced cerebral blood flow with the normal growth of age in order to maintain resultant cerebral metabolic rate of oxygen. However, the OEF did not change with age in most white matter brain regions, but decreased with age in only a few white matter brain regions. It may be related to low white matter metabolism and myelin development, the changes of blood flow and oxygen metabolism are less affected by age.Conclusion
This study suggests that QQ-based OEF mapping may serve as a useful tool to study region distinctive OEF association with age, including positive associations of OEF with age in grey matter within SomMot and SalVentAttn functional networks, but negative relationships in white matter of callosum, cingulate, superior frontal blade. The challenge free OEF mapping may be readily used to study important metabolic changes over aging-related neurodegeneration.Acknowledgements
No acknowledgement found.References
[1] DE VIS J B, HENDRIKSE J, BHOGAL A, et al. Age-related changes in brain hemodynamics; A calibrated MRI study[J]. HUM Brain MAPP, 2015, 36(10): 3973-3987.
[2] ZHANG S, CHO J, NGUYEN T D, et al. Initial experience of Challenge-Free MRI-Based Oxygen extraction fraction mapping of ischemic stroke at various stages: comparison with perfusion and diffusion mapping[J]. Front Neurosci, 2020, 14: 535441.
[3] MA Y, SUN H, CHO J, et al. Cerebral OEF quantification: A comparison study between quantitative susceptibility mapping and dual-gas calibrated BOLD imaging[J]. MAGN Reson MED, 2020, 83(1): 68-82.
[4] CHO J, SPINCEMAILLE P, NGUYEN T D, et al. Temporal clustering, tissue composition, and total variation for mapping Oxygen extraction fraction using QSM and quantitative BOLD[J]. MAGN Reson MED, 2021, 86(5): 2635-2646.
[5] CHO J, KEE Y, SPINCEMAILLE P, et al. Cerebral metabolic rate of Oxygen (CMRO(2) ) mapping by combining quantitative susceptibility mapping (QSM) and quantitative blood oxygenation level-dependent imaging (qBOLD)[J]. MAGN Reson MED, 2018, 80(4): 1595-1604.
[6] HAMPEL H, MESULAM M, CUELLO A C, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease[J]. Brain, 2018, 141(7): 1917-1933