2935
Frequency-selective pTx pulses for 3D echo planar imaging at ultra-high field
Arun Joseph1,2,3, Patrick Liebig4, Gabriele Bonanno1,2,3, Jin Jin5,6, Kurt Majewski7, Tobias Kober8,9,10, and Tom Hilbert8,9,10
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, sitem-insel AG, Bern, Switzerland, 3Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Bern, Switzerland, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Siemens Healthcare Pty Ltd, Brisbane, Australia, 6ARC Training Centre for Innovation in Biomedical Imaging Technology, The University of Queensland, Brisbane, Australia, 7Siemens AG, Munich, Germany, 8Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 9Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 10LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, sitem-insel AG, Bern, Switzerland, 3Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Bern, Switzerland, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Siemens Healthcare Pty Ltd, Brisbane, Australia, 6ARC Training Centre for Innovation in Biomedical Imaging Technology, The University of Queensland, Brisbane, Australia, 7Siemens AG, Munich, Germany, 8Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 9Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 10LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
Echo planar imaging is an important MR technique used for different applications in both structural and functional imaging. It is typically used with fat suppression to avoid phase inconsistencies induced by the chemical shift of the fat signal. pTx pulses are used to mitigate the B1+ bias field occurring at higher fields strengths; typical pTx pulses do however not suppress the fat signal. In this work, we designed a frequency-selective water excitation pTx pulse to be used in 3D EPI acquisitions, enabling homogenous, high-resolution whole-brain fat-free imaging at 7T.
Introduction
Echo planar imaging (EPI)1 has been an important tool for accelerated imaging of the whole brain since its introduction. Two- or three-dimensional segmented EPI acquisitions have been combined with different contrast mechanisms and have found many applications in both structural and functional imaging such as functional MRI (fMRI), diffusion-weighted imaging, T2*-weighted imaging2-3. The EPI sequence typically samples k-space with a bipolar readout which can result in phase in-consistencies due to the varying direction of the chemical shift, e.g., from fat. Therefore, EPI is typically used in conjunction with a fat suppression technique, e.g., spectral fat saturation or frequency-selective water excitation pulses.Imaging at higher magnetic field strengths is advantageous because of a higher signal-to-noise ratio. Albeit this enables higher resolutions4, transmit RF field (B1+) inhomogeneities are introduced. Although there are some methods to mitigate B1+ inhomogeneities such as the use of dielectric pads, the most effective solution is employing parallel transmit (pTx) pulses5 to obtain a homogenous excitation within the entire field of view.
Here, we design a frequency-selective water excitation pTx pulse to be used in 3D EPI acquisitions enabling homogenous, high-resolution, whole-brain, and fat-free imaging at 7T.
Methods
kT-points6-type excitation pulses were designed using an interior point optimizer7. The optimizer was used to minimize the difference between a target flip angle and the simulated excitation profile based on B1+ and B0 maps acquired prior to the EPI sequence. To obtain a water excitation pulse, the cost function of the optimizer was modified to also minimize the flip angle at an off-resonance frequency of -1040Hz. Other constraints were specific absorption rate (surrogate limit of 10 J/kg), hardware limitations (max RF amplitude 200 V), eleven sub-pulses, and a maximum pulse duration of 10 ms. All pulse optimizations were performed offline using MATLAB (The MathWorks Inc., Natick, MA) in conjunction with own software calling IpOpt8.All measurements were performed at 7T (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany) using an 8-channel transmit, and 32-channel receive head coil (Nova Medical, Wilmington, USA). The first experiment was performed on a phantom consisting of a bottle filled with baby oil within another bottle filled with water. In addition, another bottle filled with NiO2-doped water was also placed in the coil to have sufficient coil loading. In the second experiment, two healthy subjects were scanned for proof of concept after obtaining written informed consent.
For all experiments, a prototype 3D EPI sequence in four different pulse configurations was acquired:
- Proposed frequency-selective kT-points water excitation pulse (pTx Water)
- Non-selective kT-points excitation pulse (pTx Water + Fat)
- Slab-selective single-transmit sinc excitation pulse (1Tx Water + Fat)
- Slab-selective single-transmit sinc excitation pulse in combination with a spectral fat saturation module (1Tx Water)
Results and Discussion
Figure 1 shows the frequency-selective water excitation pTx pulse generated from the B0 and B1+ maps obtained from a volunteer. Figure 2 shows the corresponding simulated flip angle distribution on a volunteer for frequency-selective water excitation; 0Hz representing the water component and -1040Hz representing the fat component. The obtained flip angle distribution in water is homogenous and consistent with the intended target (8˚) for most regions of the brain while fat stays mostly un-excited (flip-angles up to 1° except at border). Figure 3 shows the acquired images from the phantom experiment. No fat signal is visible in the images obtained with the proposed water excitation pTx pulse, while small residual fat signal can be seen in the single transmit acquisition with spectral fat saturation. Figure 4 shows the comparison of 1Tx and pTx pulses on a volunteer. In general, the acquisitions obtained with pTx pulses show a more homogenous intensity distribution in comparison to the 1Tx acquisitions, notably in the cerebellum. Further, images acquired with water excitation pTx pulses do not have fat signal as indicated in the skull regions. Additionally, the image acquired with the spectral fat saturation shows a change in contrast (white matter is darker) which is potentially due to additional pulse power introducing magnetization transfer effects. In future work, these acquisitions need to be validated in a larger cohort including patients as well as fMRI validation experiments.Conclusion
We implemented a water excitation pTx pulse and combined it with 3D EPI acquisitions for high-resolution, whole-brain imaging without fat signal at 7T. Phantom and in vivo experiments showed a homogenous signal intensity distribution and successful suppression of the fat signal in the images. The designed pulse may improve future image quality in various applications of structural and functional imaging.Acknowledgements
No acknowledgement found.References
- Stehling MK, Turner R, Mansfield P. Echo-Planar Imaging: Magnetic Resonance Imaging in a Fraction of a Second. Science. 1991; 254(5028): 43-50.
- Turner R, Le Bihan D, Maier J, et al. Echo-planar imaging of intravoxel incoherent motion. Radiology. 1990; 177(2): 407-414.
- Turner R, Le Bihan D, Chesnicks AS. Echo-planar imaging of diffusion and perfusion. Magn Reson Med. 1991; 19(2): 247-253.
- Poser BA, Koopmans PJ, Witzel T, et al. Three dimensional echo-planar imaging at 7 Tesla. NeuroImage 2010; 51(1): 261-266.
- Katscher U, Börnert P. Parallel RF transmission in MRI. NMR in Biomed 2006; 19(3): 393-400.
- Cloos MA, Boulant N, Luong M, et al. kT-points: short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson Med. 2012; 67(1): 72-80.
- Majewski K. Simultaneous optimization of radio frequency and gradient waveforms with exact Hessians and slew rate constraints applied to kT-points excitation. Journ Magn Reson, 2021; 326,106941.
- Wächter, A., Biegler, L. On the implementation of an interior-point filter line-search algorithm for large-scale nonlinear programming. Math. Program. 2006; 106: 25–57.
Figures
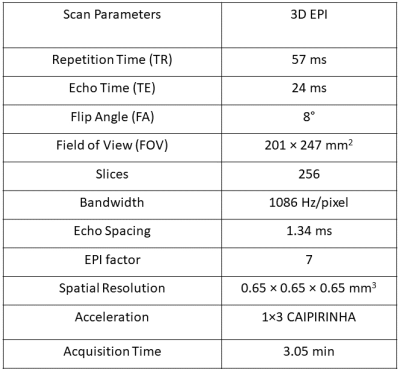
Table 1:
Scan parameters used for 3D
echo planar imaging (EPI) with different pulse configurations.
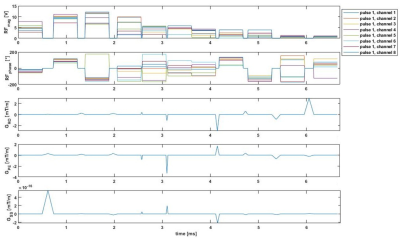
Figure 1:
Frequency-selective water excited pTx pulses generated using the B0 and B1+
maps obtained from a volunteer.
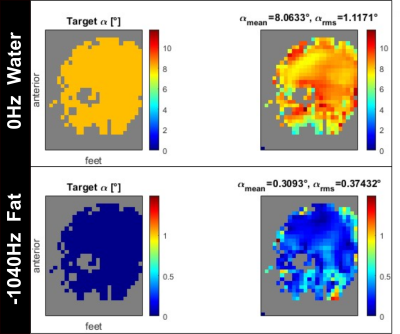
Figure 2:
Simulated flip angle distribution on a
volunteer for frequency-selective water excitation; 0Hz representing the water component
and -1040Hz representing the fat component.
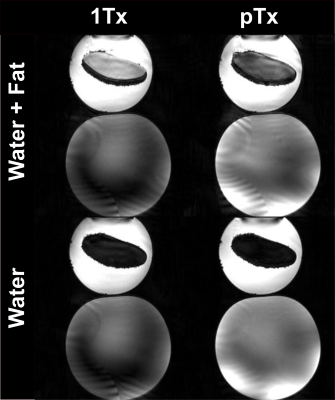
Figure 3:
Images from the phantom experiment with two water bottles, while one
bottle contains another bottle filled with baby oil. (Top) images obtained from
1Tx without fat saturation and non-selective pTx acquisitions. (Bottom) images
obtained from 1Tx slab-selective excitation with fat saturation and frequency-selective
water excitation pTx acquisitions.

Figure 4:
Images from a volunteer with pTx and
1Tx excitation pulse acquisitions. (Top) images obtained from 1Tx without fat
saturation and non-selective pTx acquisitions. (Bottom) images obtained from
1Tx slab-selective excitation with fat saturation and frequency-selective water excitation pTx
acquisitions.
DOI: https://doi.org/10.58530/2022/2935