2922
Understanding the vasodilatory effects of oxygen in oxygen enhanced functional lung MRI at 0.55T1Pulmonary Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
Oxygen-enhanced lung MRI is promising for assessing regional lung function (ventilation and perfusion) but the physiological contributions to signal enhancement are poorly characterized. We sought to assess how oxygen-induced pulmonary vasodilation may contribute to signal enhancement by altering pulmonary blood volume and therefore lung proton density. We acquired repeated PDw and T1w images using a 0.55 T prototype MRI system in anesthetized pigs exposed to room air, 100% O2, 50% O2, hypoxia and experimental pulmonary hypovolemia. Our findings suggest that T1w signal intensity is affected by oxygen through both T1 shortening and vascular activity.
Introduction
Oxygen-enhanced (OE) magnetic resonance imaging (MRI) is a promising tool for assessing regional lung function.1-3 Potential uses of OE-MRI include diagnosis, risk-stratification and treatment monitoring of asthma and chronic obstructive pulmonary disease.2,4 OE-MRI uses inhaled 100% oxygen as a T1 shortening contrast agent, and the resulting local signal enhancement is assumed to represent a combination of ventilation and perfusion. The T1 relaxivity of oxygen is higher at lower field strengths. However, oxygen is also a vasodilator in the lungs, and it is anticipated that inhalation of 100% oxygen will cause vasodilation. Therefore, local blood flow may cause additional signal intensity changes in OE-MRI.Here, we sought to characterize the contributions of oxygen induced global vasodilation on OE-MRI using T1-weighted (T1w) and proton density weighted (PDw) using various experimental conditions in animals.
Methods
We imaged 12 anesthetized female pigs (weight: 47±8) using a prototype 0.55T high-performance MRI system5, exposed sequentially to either:A: Baseline room air, 100% O2, 50% O2, Repeat room air and hypoxia (n=6),
or
B: Baseline room air, 100% O2 and partial occlusion of the inferior vena cava (IVC) (n=6).
Hypoxia and partial IVC occlusion experimental states were used to induce pulmonary vasoconstriction and reduced blood volume, respectively. Partial IVC occlusion was maintained for 20-30 minutes with periodic adjustment of balloon inflation to achieve a stable decrease in systemic systolic blood pressure to 50-65 mmHg from a typical baseline of ~80 mmHg. Prior studies suggest that oxygen affects vascular tone most between hypoxia-normoxia at 50-100 mmHg PaO2. 6,7 Therefore, OE-MRI signal may be disproportionately affected by O2-induced vasodilation in the presence of local or global hypoxia.
We obtained the following measurements in each state: arterial/venous blood gases, invasive systemic and pulmonary blood pressures, fraction of inspired oxygen (FiO2) and pulse oximeter saturation.
The following images were acquired on a prototype 0.55T MRI system (MAGNETOM Aera, Siemens, Erlangen, Germany) in each experimental state:
1. Two T1w acquisitions of the lung (prototype 3D free-breathing gradient echo UTE-sequence, stack-of-spirals trajectory, TR: 6.5 ms, TE: 0.04 ms, FA: 13 degrees, acquisition time ~4.5 minutes),
2. Two PDw acquisitions of the lung (3D UTE, TR: 6.5 ms, TE: 0.04 ms, FA: 1 degree, acquisition time ~4 minutes),
3. 2D main pulmonary arterial flow measurement.
4. T1 mapping (Modified look-locker inversion recovery (MOLLI)) in a cardiac 4-chamber view.
T1w and PDw images were reconstructed at a stable respiratory phase.8 Percent signal enhancement (PSE) maps were calculated using relative signal intensity differences from baseline room air following image registration9. Lungs were manually segmented and the median PSE value of the whole lungs was reported. For the baseline room air state, the second versus first T1w and PDw acquisition was used to derive reported median PSE values. For all other states, the average median PSE from the two T1w or two PDw acquisitions was reported. To avoid capturing excessive variation in proton density caused by respiratory variation, we rejected acquisitions in which lung volume deviated >10% from the baseline room air reference images.10
Results
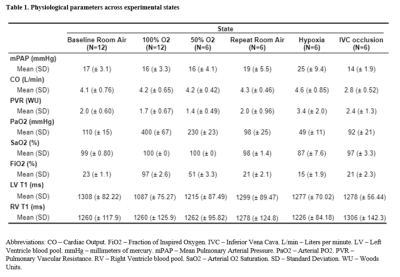
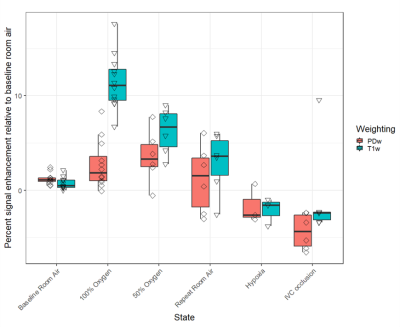
The median PSE value per experimental state is shown in Figure 1. A total of 6 PDw and 7 T1w acquisitions were rejected due to >10% lung volume variation from the baseline room air reference images. Signal enhancement in T1w images exhibited a dose-response relationship with FiO2, whereas the signal enhancement in PDw images was similar in 50% and 100% FiO2. Apart from one outlier, both PDw and T1w images showed negative enhancement during both hypoxia and IVC occlusion, compared with the baseline room air acquisition. Repeated measurements during room air inhalation suggests reduced repeatability with extended duration between measurements.Hemodynamic parameters and measured T1 from the left and right ventricular blood pools are provided in Table 1. Arterial pO2 confirms increased oxygenation with greater FiO2. Mean pulmonary arterial pressure (mPAP) rose sharply in hypoxia, confirming hypoxic vasoconstriction. Cardiac output (CO) and mPAP were both lower during IVC occlusion while pulmonary vascular resistance (PVR) rose slightly, suggesting successful induction of pulmonary hypovolemia through decreased venous return.
Discussion
Our findings suggest that oxygen-induced changes in pulmonary blood volume provide a small contribution to T1w signal enhancement. The negative median PDw and T1w enhancement in both hypoxia and partial IVC occlusion suggests that both sequences were sensitive to variations in pulmonary blood volume. Correspondingly, the observed positive median enhancement of PDw images in hyperoxia suggests a component of vasodilation contributing to oxygen induced MRI signal enhancement. Collectively, these findings indicate the potential sensitivity of OE-MRI to vascular dysfunction.A correction for differences in lung volume between experimental states was not implemented9. We only studied a limited number of pigs anesthetized with isoflurane, which is a vasodilator itself, hampering our ability to draw conclusions about a clinical population. Further work is required to generate a correction for vasodilation on the individual-level.
Conclusions
Our results suggest that oxygen-induced pulmonary vasodilation may contribute to T1w OE-MRI images used to assess lung function.Acknowledgements
Authors BW and FS contributed equally to this work. The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between NHLBI and Siemens Healthcare. The study was supported by the Intramural Research Program, NIH/NHLBI (Z01-HL006257, Z01-HL006213, Z01-HL006039).References
1. Edelman RR, Hatabu H, Tadamura E, Li W, Prasad PV. Noninvasive assessment of regional ventilation in the human lung using oxygen-enhanced magnetic resonance imaging. Nat Med. Nov 1996;2(11):1236-9.
2. Jobst BJ, Triphan SM, Sedlaczek O, et al. Functional lung MRI in chronic obstructive pulmonary disease: comparison of T1 mapping, oxygen-enhanced T1 mapping and dynamic contrast enhanced perfusion. PLoS One. 2015;10(3):e0121520. doi:10.1371/journal.pone.0121520
3. Ohno Y, Nishio M, Koyama H, et al. Asthma: comparison of dynamic oxygen-enhanced MR imaging and quantitative thin-section CT for evaluation of clinical treatment. Radiology. Dec 2014;273(3):907-16. doi:10.1148/radiol.14132660
4. Zha W, Kruger SJ, Johnson KM, et al. Pulmonary ventilation imaging in asthma and cystic fibrosis using oxygen-enhanced 3D radial ultrashort echo time MRI. J Magn Reson Imaging. 05 2018;47(5):1287-1297. doi:10.1002/jmri.25877
5. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 11 2019;293(2):384-393. doi:10.1148/radiol.2019190452
6. Hambraeus-Jonzon K, Bindslev L, Mellgård AJ, Hedenstierna G. Hypoxic pulmonary vasoconstriction in human lungs. A stimulus-response study. Anesthesiology. Feb 1997;86(2):308-15. doi:10.1097/00000542-199702000-00006
7. Dunham-Snary KJ, Wu D, Sykes EA, et al. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest. Jan 2017;151(1):181-192. doi:10.1016/j.chest.2016.09.001
8. Javed A, Ramasawmy R, O'Brein K, et al. Self-gated 3D Stack-of-Spirals Ultra-Short Echo-Time Pulmonary imaging at 0.55T. Magnetic Resonance in Medicine [in press]. 2021;
9. Guetter C, Xue H, Chefd'hotel C, Guehring J. Efficient symmetric and inverse-consistent deformable registration through interleaved optimization. 2011:590-593.
10. Bhattacharya I, Ramasawmy R, Javed A, et al. Oxygen-enhanced functional lung imaging using a contemporary 0.55 T MRI system. NMR Biomed. 08 2021;34(8):e4562. doi:10.1002/nbm.4562