2921
A semi-realistic and reusable 3D printed brain phantom for MR-based Electrical Properties Tomography1Department of Radiotherapy, Division of Imaging and Oncology, University Medical Center Utrecht, Utrecht, Netherlands, 2Computational Imaging Group for MR Diagnostics and Therapy, Center for Image Sciences, University Medical Center Utrecht, Utrecht, Netherlands, 3Department of Orthopaedics, University Medical Center Utrecht, Utrecht, Netherlands, 4Department of Clinical Sciences, Faculty of Veterinary Medicine, Utrecht University, Utrecht, Netherlands
Synopsis
This work presents a semi-realistic and reusable 3D printed brain phantom to benchmark MR-Electrical-Properties-Tomography reconstruction methods. We show that the hollow compartments of this phantom can be refilled multiple times with different water-based solutions of known electrical properties, which are otherwise not available from in-vivo measurements. Additionally, these brain phantoms can be used inside electromagnetic simulation software, allowing for MR-EPT reconstructions in controlled, simulation settings. In this way, a database comprising of simulated and measured data using these brain models and corresponding 3D printed phantoms will be generated and shared for the first MR-EPT reconstruction challenge.
Introduction
In MR-based electrical properties tomography (EPT), electrical properties (EPs, conductivity σ and relative permittivity εr) are derived from non-invasive MR measurements. Several reconstruction methods have been presented, however the reported in-vivo results often lack agreement1,2.Benchmarking of in-vivo EPs reconstructions is hampered by the lack of knowledge of ground-truth (GT) EPs values. Therefore, these reconstruction methods are often evaluated on phantom measurements, where GT-EPs values are available. However, by using simplified phantoms (e.g. spheres/cylinders), the validity of certain assumptions made by the reconstruction approaches (e.g. symmetry) and the impact of realistic tissue structures (e.g. tissue boundaries) may not be thoroughly studied3,4.
In this work, we present the first 3D printed, brain-like, MR compatible phantom, in which white/gray matter and cerebrospinal fluid structures can be filled independently with liquids of known EPs. This will allow benchmarking of MR-EPT reconstruction algorithms in a more realistic scenario. Additionally, we show that the 3D printed, brain-like geometry can be easily imported in electromagnetic (EM) simulation software for EPT reconstructions on in-silico data. This allows direct comparison between reconstruction performance in ideal (simulations) and realistic (MR) settings. All the measured and simulated data that will be generated with these phantoms/models will be used to create a database that will be shared with the EPT community to favor benchmarking of EPT reconstruction algorithms.
Materials and Methods
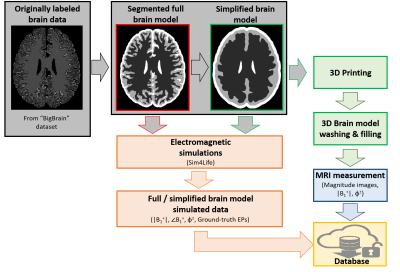
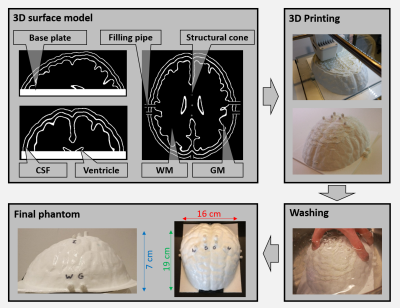
The overall workflow describing the procedure to go from brain MRI data to a 3D printed brain-like phantom, and the creation of the MR-EPT database comprising of MRI measurements and EM simulations is shown in Fig.1. The brain data was taken from the BigBrain dataset5 (voxels labeled as white matter (WM), gray matter (GM) and cerebrospinal fluid (CSF)). Using 3DSlicer6, the segmented model was modified (smoothing, expansion, island filling and logical operations) to create a model with smooth surfaces and 4 compartments: WM, GM, CSF, and ventricles. The obtained 3D model was used for both EM simulations and 3D printing.Structural cylinders were added to the surface model to prevent structural breakdown (Fig.2). To make all compartments fillable, various pipes and holes were added. The 3D model was imported in Cura7 for slicing and conversion to GCode and printed via fuse deposition modelling on an Ultimaker S3 3D printer (layer height 0.2mm). Surfaces were printed with polylactic acid (PLA), while a water-soluble support material polyvynil alcohol (PVA) was used to enable printing of overhanging structures. Printing time was about 2 days, after which the phantom was put in a water-bath for 2 days to wash out the support material. The compartments of the phantom were then filled with water/gelatin-based solutions (Fig.38).
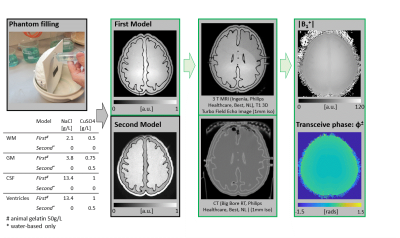
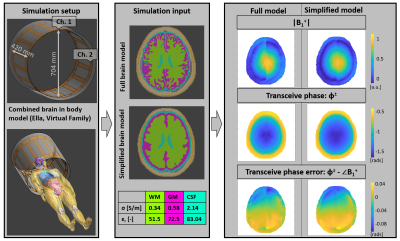
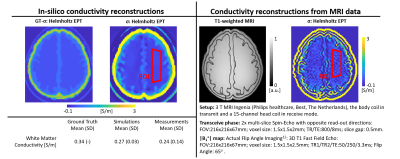
MRI measurements were performed on a clinical 3 T MRI system (Fig.3). The original brain model and the simplified one (used for printing) were also used for EM simulations in Sim4Life9 (Fig.4: simulation setup and EM fields).
Helmholtz-EPT reconstructions were performed on both measured and simulated data.
Results and Discussion
From the contrast difference in the T1-weighted images of Fig.3, it can be seen that the compartments of the phantom can be individually filled, washed, and refilled. This allows the creation of a database of measured data using the same 3D printed phantom model but different, known, EPs values. This can be exploited for training deep-learning methods for EPT reconstructions (otherwise difficult for in-vivo data due to lack of GT-EPs knowledge10). For illustration, B1+ magnitude and transceive phase maps obtained from MRI measurements using this 3D printed phantom are shown.From the performed measurements, we did not observe any imaging artifact created by the phantom or the materials used for its construction. A few bubbles were left within the gelatin, which may be avoided by slower phantom filling. A small leakage from the phantom surface was also observed. This issue may be overcome by increasing the printing resolution, temperature and thickness of the outer layer.
In Fig.4, simulated EM fields illustrate that the model simplification does not have a major effect on the resulting fields.
In Fig.5, conductivity maps reconstructed from simulated (controlled settings) and measured (realistic settings) data using the same phantom are shown, demonstrating similar quality and, therefore, the feasibility of using a 3D printed brain phantom for benchmarking EPT reconstructions.
By using the same phantom model, EPT reconstructions from EM simulations and MR measurements can be directly compared. Also, measurement related reconstruction issues of different MR-EPT methods can be accurately characterized. Furthermore, this allows testing the (noise) robustness and generalizability of emerging deep-learning EPT methods (trained on simulated data) with realistic MR measurements on brain models with known GT-EPs values (otherwise not available for in-vivo data).
The measured and simulated data generated with this type of phantoms will be shared with the EPT community allowing future reproducibility and standardization studies, together with the first MR-EPT reconstruction challenge.
Conclusion
A reusable, 3D printed, brain-like, MR compatible phantom is presented for the first time for benchmarking MR-EPT reconstructions from MRI measurements as this allows knowledge of GT-EPs. All the measured/simulated data obtained from such brain models/3D printed phantoms will be shared with the MR-EPT community in the context of the first MR-EPT challenge.Acknowledgements
This work was supported by the Netherlands Organisation for Scientific Research (NWO-Veni), grant number:18078.References
1. McCann, Hannah, Giampaolo Pisano, and Leandro Beltrachini. "Variation in reported human head tissue electrical conductivity values." Brain topography 32.5 (2019): 825-858. Original manuscript
2. Mandija, Stefano, et al. "Brain Tissue Conductivity Measurements with MR-Electrical Properties Tomography: An In Vivo Study." Brain topography 34.1 (2021): 56-63.
3. Duan, Song, et al. "Quantitative analysis of the reconstruction errors of the currently popular algorithm of magnetic resonance electrical property tomography at the interfaces of adjacent tissues." NMR in Biomedicine 29.6 (2016): 744-750.
4. Mandija, Stefano, et al. "Error analysis of helmholtz‐based MR‐electrical properties tomography." Magnetic resonance in medicine 80.1 (2018): 90-100.
5. Amunts K, Lepage C, Borgeat L, Mohlberg H, Dickscheid T, Rousseau M-É, Bludau S, Bazin PL, Lewis LB, Oros-Peusquens AM, Shah NJ, Lippert T, Zilles K, Evans AC. BigBrain: An ultrahigh-resolution 3D human brain model. Science. 2013; 340(6139):1472-1475. doi: 10.1126/science.1235381. PMID: 23788795.
6. Fedorov, Andriy, et al. "3D Slicer as an image computing platform for the Quantitative Imaging Network." Magnetic resonance imaging 30.9 (2012): 1323-1341.
7. Ultimaker, “Cura 3D Printing Slicing Software,” (Accessed: 2021-10-26). [Online]. Available: https://ultimaker.com/en/products/cura-software
8. Duan, Qi, et al. "Characterization of a dielectric phantom for high‐field magnetic resonance imaging applications." Medical physics 41.10 (2014): 102303.
9. Sim4Life (ZMT AG, Zurich, CH)
10. Hampe, Nils, et al. "Investigating the challenges and generalizability of deep learning brain conductivity mapping." Physics in Medicine & Biology 65.13 (2020): 135001.
11. Gosselin M C, Neufeld E, Moser H, Huber E, Farcito S, Gerber L, Jedensjö M, Hilber I, Di Gennaro F, Lloyd B, Cherubini E, Szczerba D, Kainz W, Kuster N, Development of a new generation of high-resolution anatomical models for medical device evaluation: the Virtual Population 3.0, Physics in Medicine and Biology, 59(18):5287-5303, 2014.
12. Yarnykh, Vasily L. "Actual flip‐angle imaging in the pulsed steady state: a method for rapid three‐dimensional mapping of the transmitted radiofrequency field." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 57.1 (2007): 192-200.
Figures