2916
Robust measurements of current-induced magnetic fields in the human brain by EPI1Section for Magnetic Resonance, DTU Health Tech, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital - Amager and Hvidovre, Copenhagen, Denmark, 3Sino-Danish College, University of Chinese Academy of Sciences, Beijing, China, 4High-Field Magnetic Resonance Center, Max-Planck-Institute for Biological Cybernetics, Tübingen, Germany, 5State Key Laboratory of Brain and Cognitive Science, Beijing MRI Center for Brain Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 6Center for Excellence in Brain and Science and Intelligence Technology, Chinese Academy of Sciences, Beijing, China
Synopsis
Magnetic resonance current density imaging (MRCDI) can measure the magnetic fields created in the human brain from currents injected via surface electrodes. Previous methods have demonstrated high sensitivity sufficient for low current strengths (~1 mA). However, they have also proven susceptible to physiological noise. Here we increase the temporal resolution of the method and thereby the robustness to physiological noise by using echo-planar imaging (EPI) for the acquisition. We show that the method produces reliable magnetic field measurements with an average sensitivity of 52 pT for a 2 minutes scan with 3 mm isotropic resolution.
Introduction
Computational volume conductor models of the human head are increasingly used in neuroscientific research to estimate electric fields induced by non-invasive brain stimulation methods (e.g. TES or TMS) or for source localization in electro- and magnetoencephalography. Subject-specific head models can be created from structural MR images1. However, creating accurate and reliable head models is challenging due to the complex anatomy of the human head. Magnetic resonance current density imaging (MRCDI) is a great candidate tool for validating volume conductor models and estimating tissue conductivities. MRCDI measures small perturbations to the phase of the complex MR image caused by the magnetic fields from currents injected via surface electrodes2. Previous human in-vivo brain MRCDI studies have used multi-echo gradient-echo (MGRE) sequences3–6. In the studies where control measurements with zero current (noise floor measurements) were reported5,6, low-frequency spatial noise was observed in the MRCDI data, most likely caused by physiological noise. In this study, we increase the robustness to physiological noise by using an echo-planar imaging (EPI) sequence with a high temporal resolution for MRCDI measurements. We also compare the results to simulated magnetic fields in a subject-specific volume conductor model.Methods
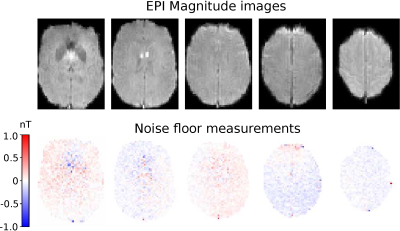
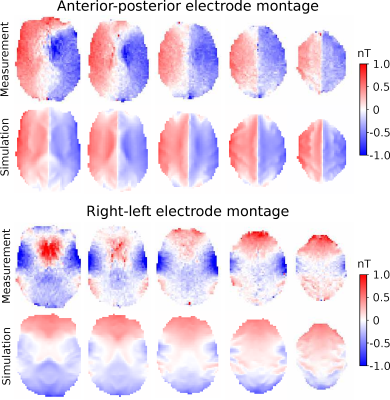
The experiments were performed on a 3T MR scanner (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) with a 64 channel head coil. A double-echo EPI sequence provided by the Center for Magnetic Resonance Research (CMRR, Minneapolis, Minnesota, USA) was used to reduce geometric distortions. The sequence parameters were TR = 120 ms, TE = 25.6, 63.48 ms, Flip angle 30°, FOV 225x190 mm2, matrix size 76x64, and ~3 mm isotropic resolution. Five slices were acquired with 1000 measurements for each slice, resulting in two minutes of acquisition time per slice and a total acquisition time of 10 minutes. First, a wire loop experiment was conducted for one subject to verify the accuracy of the measured magnetic field by the EPI-based MRCDI sequence. A conducting rubber wire7 was placed around the subject’s head with a 2 mA current alternating in synchrony with the sequence. The magnetic field caused by the current in the wire loop is measurable in the human brain by MRCDI. The wire was tracked with an ultrashort echo time sequence and the Biot-Savart law was used to simulate the current-induced field in the imaged brain slices. This was subtracted from the magnetic fields measured by the MRCDI sequence. Noise floor measurements were also conducted to quantify the noise in the EPI-based MRCDI measurements. Four subjects were measured without injected currents. The noise level for each subject and each imaged slice was defined as the standard deviation of the magnetic field measurements from the current-free images. For the last experiment, 1mA currents were injected into the subject’s brain via surface electrodes7. Data were acquired from four subjects and two electrode montages: one anterior-posterior (AP) and one right-left (RL) montage. For each subject, a volume conductor model was created and magnetic fields were simulated for the respective electrode montages using the finite element method implemented in SimNIBS8. The difference between measurements and simulations was quantified as the relative root mean square (RRMS) difference.Results
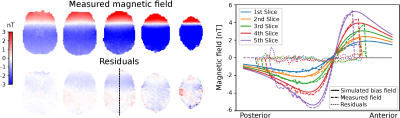
The data from the loop experiment is presented in figure 1. It is clear that the EPI sequence produces reliable magnetic field measurements. Weak residual errors are still present, which could be caused by inaccurate localization of the wire or subject movement during scanning. The noise floor measurements for one subject are presented in figure 2 and summarized in table 1 for all subjects. A sensitivity of 52 pT was achieved on average for all subjects and all imaged slices with very low variability between subjects. MRCDI measurements with injected currents from one subject are shown in figure 3. The similarity between measured and simulated magnetics fields is clear for the AP montage, while there is a larger discrepancy for the RL montage. The RRMS difference between measurement and simulation for all subjects is presented in table 2. There is consistently a larger discrepancy between measurements and simulation for the RL electrode montage. The consistent results indicate that MRCDI can reliably map current-induced magnetic fields in the human brain and guide improvements of computational volume conductors as well as estimate tissue conductivities.Conclusion
We have shown that dual-echo gradient-echo EPI is a good candidate for high temporal resolution and physiological noise-robust MRCDI. Consistently larger differences between measurements and simulations for the RL montage compared to the AP montage indicate that the computational head models have to be improved and that MRCDI is a good candidate for the task.Acknowledgements
This study was supported by the Lundbeck Foundation (grants R313-2019-622 and R244-2017-196 to AT; R324-2019-1784 to LGH), the Chinese National Major Scientific Equipment R&D Project (grant ZDYZ2010-2), and a PhD stipend of the Sino-Danish Center for Education and Research to FG.References
1. Nielsen JD, Madsen KH, Puonti O, et al. Automatic skull segmentation from MR images for realistic volume conductor models of the head: Assessment of the state-of-the-art. Neuroimage. 2018;174(August 2017):587-598. doi:10.1016/j.neuroimage.2018.03.001
2. Joy M, Scott G, Henkelman M. In vivo detection of applied electric currents by magnetic resonance imaging. Magn Reson Imaging. 1989;7(1):89-94. doi:10.1016/0730-725X(89)90328-7
3. Kasinadhuni AK, Indahlastari A, Chauhan M, Schär M, Mareci TH, Sadleir RJ. Imaging of current flow in the human head during transcranial electrical therapy. Brain Stimul. 2017;10(4):764-772. doi:10.1016/j.brs.2017.04.125
4. Chauhan M, Indahlastari A, Kasinadhuni AK, Schar M, Mareci TH, Sadleir RJ. Low-Frequency Conductivity Tensor Imaging of the Human Head In Vivo Using DT-MREIT: First Study. IEEE Trans Med Imaging. 2018;37(4):966-976. doi:10.1109/TMI.2017.2783348
5. Göksu C, Hanson LG, Siebner HR, Ehses P, Scheffler K, Thielscher A. Human in-vivo brain magnetic resonance current density imaging (MRCDI). Neuroimage. 2018;171(December 2017):26-39. doi:10.1016/j.neuroimage.2017.12.075
6. Göksu C, Scheffler K, Gregersen F, et al. Sensitivity and resolution improvement for in vivo magnetic resonance current‐density imaging of the human brain. Magn Reson Med. 2021;(March):1-16. doi:10.1002/mrm.28944
7. Gregersen F, Göksu C, Schaefers G, Xue R, Thielscher A, Hanson LG. Safety evaluation of a new setup for transcranial electric stimulation during magnetic resonance imaging. Brain Stimul. 2021;14(3):488-497. doi:10.1016/j.brs.2021.02.019
8. Thielscher A, Antunes A, Saturnino GB. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2015;2015-Novem:222-225. doi:10.1109/EMBC.2015.7318340
Figures