2909
Disentangling apparent discordance between ASL-MRI and [18F]-FDG PET following a single dose of the β2-agonist clenbuterol1Invicro, London, United Kingdom, 2Center for Clinical Pharmacology, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium, 3Division of Nuclear Medicine, University Hospital Leuven, Leuven, Belgium, 4Nuclear Medicine and Molecular Imaging, KU Leuven, Leuven, Belgium, 5Department of Neurology, University Hospital Leuven, Leuven, Belgium, 6Centre for Neuroimaging Sciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College, London, United Kingdom, 7CuraSen Therapeutics, San Carlos, CA, United States
Synopsis
Here we demonstrate that an apparent discrepancy in CBF (from ASL) and CMRglu (from [18F]-FDG PET) following high-dose administration of clenbuterol in healthy volunteers is caused by within-scan rising plasma glucose concentrations. With simulation-based correction, post-clenbuterol CBF changes from ASL agree with blood flow estimates from [18F]-FDG PET, without increase in CMRGlu. ASL-MRI may therefore provide a valuable tool for monitoring the central effects of β2-adrenergic receptor activation in larger, future studies on both healthy volunteers and patients with neurodegenerative disorders.
Introduction
Activation of the noradrenergic system via administration of β2-adrenergic receptor (β2-AR) agonists could offer therapeutic benefit in neurodegenerative disorders that suffer early-stage deficiency of this system1. Prior studies found increases in cerebral blood flow (CBF) with salbutamol in healthy volunteers2, as well as with clenbuterol in patients with neurodegenerative diseases3. However, apparent discrepancies in CBF and brain metabolic activity (CMRglu) were observed in healthy volunteers at the highest dose of clenbuterol. Here we present our findings and apply simulations to disentangle the apparent discrepancy in the direction of change of these parameters.Methods
Four healthy volunteers (all male, mean age 46.3 years (range 41-53)) underwent single-delay 3D pCASL (TR=4854ms, TE=10.7ms, FA=111°, FOV=240x240mm, slice thickness=4mm, 36 slices, label duration = 1800ms, post-label delay=2025ms), a T1-weighted FSPGR scan (TR=6680ms, TE=2.81ms, FA=11°, FOV=256x256mm, slice thickness=1.2mm, 188 slices), and [18F]FDG-PET dynamic 60-min imaging at baseline (pre-dose), and at ~3 hours post administration of 160 µg Clenbuterol (N=3) on the first day of scanning. After a 7-day washout, subjects underwent a second day of scanning: receiving a morning 5 mg dose of the peripherally restricted β-blocker nadolol, a pre-dose MRI scan, followed by combined PET-MR imaging ~3 hours post administration of 160 µg Clenbuterol (N=4). Imaging was performed simultaneously on a GE Signa PET-MR 3T system. Regional CBF values were extracted from the system-generated CBF map, while kinetic analysis of PET data was performed in the PKIN toolbox of PMOD 3.802 to calculate the net uptake rate for [18F]FDG (Ki), the cerebral metabolic rates of glucose (CMRglu), and [18F]FDG influx in brain tissue (K1). Subjects also had blood glucose levels measured.Results
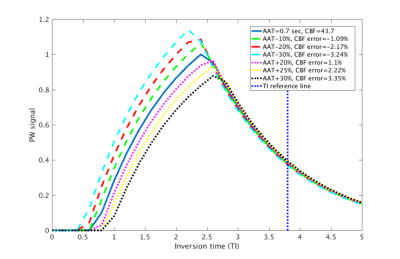
There was a localised CBF increase in the hippocampus with first day dosing of 160 µg clenbuterol, with mean relative displacement (MRD) from baseline to post-dose of 28±6% (N=3; with one subject not performing post-dose scanning due to hyperglycemia). With the same localisation but apparently opposite direction of change, there was a general decrease of CMRGlu in the hippocampus following administration of 160 µg of clenbuterol (MRD of -22±7%, N=3). However, both the plasma glucose level and the rate of inward transport of [18F]-FDG in the brain (parameter K1) did increase post-clenbuterol (28±12% and 12±18%, respectively).Using standard expressions4 and recommended parameters from the ASL consensus paper5, simulation of the perfusion-weighted signal with altered arterial arrival time (AAT, ranging from -30% to +30%; Figure 1), revealed a maximum change of CBF due to potential changes in AAT of < 4%.
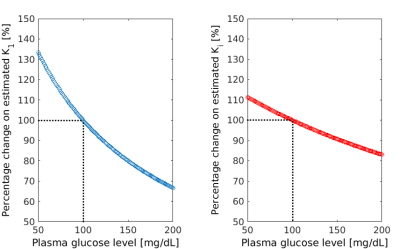
Simulation of the effect of changes in the plasma glucose level on the estimated [18F]-FDG rate constants (ranging from -50% to +100% of glucose concentration at euglycaemia, set to 100 mg/dL; Figure 2) indicated that the bias on K1 and Ki was bounded between +33% and -33% for K1, +11% and -17% for Ki. Thus, if glucose level was increasing during the 60 min scan, we would be underestimating K1 (up to -33% for an increase in glucose to 200 mg/dL). Coupled with the knowledge that delivery rate can be expressed as K1 = flow * extraction, we calculated an estimate of the CBF measured with PET (CBFPET) from the [18F]FDG PET K1; obtaining a value of 20±23%, in-line with the increase in CBF measured with ASL in same cohort. Furthermore, when correcting the metabolic rate with the predicted glucose level at the end of the scan, we found no change in CMRGlu between baseline and post-dose scans.
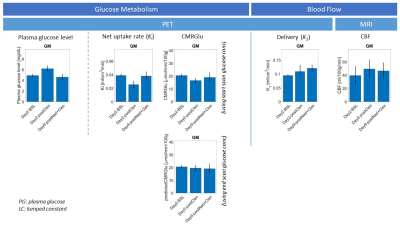
After controlling for peripheral effects with 5 mg nadolol on the second day of dosing, the discordance between ASL and PET was less pronounced (Figure 3): both CBF and plasma glucose changes were heavily modulated (with average CBF response of +13% across the eight GM ROIs), and only one subject showed a marked decrease in CMRGlu (~30% decrease across all ROIs).
Conclusion
With appreciation of the limited sample size, our findings provide initial evidence that the central effect of clenbuterol on CBF in healthy volunteers cannot be solely explained by plausible effects of the drug on arterial transit time. Furthermore, the post-clenbuterol CBF changes measured with ASL were in agreement with blood flow changes estimated from [18F]-FDG PET. After correction for rising plasma glucose levels during the scan, there was no evidence of increase in CMRGlu after administration of clenbuterol. ASL-MRI may therefore provide a valuable tool for monitoring the central effects of β2-adrenergic receptor activation in larger, future studies on both healthy volunteers and patients with neurodegenerative disorders.Acknowledgements
With thanks to the researchers and healthy volunteers who participated in this study.References
1. Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011 Nov;70(11):960-9
2. Vargas G, Bishop C, Rizzo G, et al. Beta-Adrenoceptor Agonism Evokes Acute Imaging Signals in Healthy Individuals. The 15th International Conference on Alzheimer’s and Parkinson’s Diseases and related neurological disorders, AD/PD™ 2021; March 2021.
3. Lodeweyckx T, De Hoon J, Van Laere K, et al. Safety, Tolerability and Cerebral Blood Flow After Single Doses of the β2-agonist, Clenbuterol, in Patients with Mild Cognitive Impairment or Parkinson’s Disease. 14th edition of Clinical Trials on Alzheimer’s Disease (CTAD 2021), Boston, November 9-12, 2021.
4. Wong EC, Buxton RB, Frank LR. A theoretical and experimental comparison of continuous and pulsed arterial spin labeling techniques for quantitative perfusion imaging. Magn Reson Med. 1998;40(3):348-355.
5. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102-116.
Figures