2893
MR-guided high intensity focused ultrasound of extra-abdominal desmoid tumors: a retrospective study of 61 patients
Daniel Duex1, Vipul Sheth1, Ryan Brunsing1, Kristen Ganjoo2, Raffi Avedian3, Rachelle Bitton1, and Pejman Ghanouni1
1Radiology, Stanford University, Stanford, CA, United States, 2Medicine - Med/Oncology, Stanford University, Stanford, CA, United States, 3Orthopaedic Surgery, Stanford University, Stanford, CA, United States
1Radiology, Stanford University, Stanford, CA, United States, 2Medicine - Med/Oncology, Stanford University, Stanford, CA, United States, 3Orthopaedic Surgery, Stanford University, Stanford, CA, United States
Synopsis
We reviewed MR-guided high intensity focused ultrasound treatments (MRgFUS) of extra-abdominal desmoid tumors (DTs) in our institution from 02/2013 until 12/2020. The primary endpoints for assessing efficacy are post-treatment change in tumor volume, response based on RECIST and mRECIST; secondary endpoints are change in pain and quality of life. Complications are also reported. Our study demonstrates MRgFUS is an effective treatment option for first line and salvage therapy of desmoid tumors.
Synopsis
We reviewed MR-guided high intensity focused ultrasound treatments (MRgFUS) of extra-abdominal desmoid tumors (DTs) in our institution from 02/2013 until 12/2020. The primary endpoints for assessing efficacy are post-treatment change in tumor volume, response based on RECIST and mRECIST; secondary endpoints are change in pain and quality of life. Complications are also reported. Our study demonstrates MRgFUS is an effective treatment option for first line and salvage therapy of desmoid tumors.Summary of Main Findings
MRgFUS of extra-abdominal desmoid tumors resulted in significant reduction of viable tumor after a median follow-up of 1 year, with mean progression free survival (PFS) of 20.8/9.8 months (RECIST/mRECIST). Pain and quality of life improved significantly. Main adverse events (AEs) were skins burns and nerve palsy. Severe AEs were rare.Introduction
Desmoids are locally highly aggressive soft tissue tumors (1-3), many of which show a highly progressive and recurrent pattern in mainly young patients. Although treatment strategies include observation, surgery (6, 7), radiation (8-10) or medical therapy (11-17), these approaches introduce significant morbidity, including loss of function, pathological fractures, secondary malignancies and toxicity (18). This study investigates our eight-year experience using MRgFUS for treatment of extra-abdominal desmoid tumorsMethods
Patient Selection:After approval from our institutional review board, we retrospectively reviewed patients with biopsy-proven desmoid tumors treated with MRgFUS between 02/2013 and 12/2020. All patients underwent multi-disciplinary evaluation. Patients had pre- and post-treatment MR imaging.
Data Collection and Analysis:
Data recorded through a retrospective chart review included demographic patient information, prior and additional treatments, symptoms, tumor volume and complications. Patients receiving subsequent treatment other than MRgFUS were exited from the study. Pre- and post-ablation assessment compared the total and viable tumor volume as measured by contouring the tumor on pre- and post-contrast MRI (Horos). Best and latest post-therapeutic response and progression free survival were assessed with RECIST v.1.1 (19), measuring maximal tumor diameter, and mRECIST (20), measuring maximal enhancing diameter. Complications were recorded per the Society of Interventional Radiology (SIR) Adverse Event Severity Scale (21).
MRgFUS procedure:
All therapies were performed with the ExAblate 2000 System (INSIGHTEC, Israel) coupled to a 3 Tesla MRI (GE Healthcare, USA). The desmoids and surrounding critical structures such as nerves or vessels were contoured on T2- and proton-density-weighted sequences, which the system used to generate a treatment plan; the technical parameters for each sonication were then manually optimized by the physician. Every sonication was monitored by real-time thermometry. Post-contrast images for immediate assessment of the treatment were performed.
Results
A total of 61 patients (median age: 35, range 3 – 66; 44 females) were treated with 77 sessions of MRgFUS (tumor locations in Figure 1, example treatments in Figure 2 and 3). The median tumor volume at the treatment day was 100 cm3 (range: 4.1-1940 cm3), with a median of 80 % of the targeted tumor ablated (range 6-100 %).The subsequent median total and viable tumor volume decreased to 54 cm3 (range: 0-862 cm3, p: 0,08) and 42 cm3 (range: 0-646 cm3, p: 0,002) at a median time of 1 year after treatment (range: 1-79 months). Overall best response (mRECIST) per patient was 9 CR, 27 PR, 3 SD, 15 PD (measured at a median of 8 months, range 1-93 months). Progression free survival was reached at 11 and 24 months based on mRECIST and RECIST, respectively.
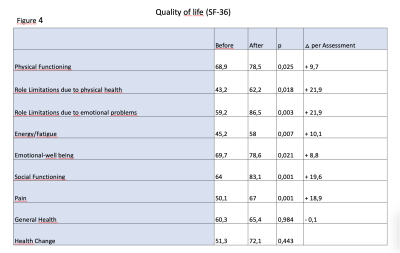
After MRgFUS, pain (when NRS mean at baseline ≥4) decreased from 5,64±1,4 to 2,66±1,8, p<0.001. Quality of Life improved significantly (Figure 4). Patients were often able to stop medical treatment (75.4 % had medical treatment before vs 52.5 % after, p=0.03).
The most common complication was skin burn, occurring in 34 % of treatments, 82 % of which were 1st or 2nd degree; this risk appears to be greater when the tumor is <1cm from the skin. The introduction of skin cooling techniques during treatment reduced the incidence by 46 % (p=0.03). Nerve palsy post-MRgFUS occurred in 12 patients, and only in patients where the nerve was ≤4 mm from the tumor. Complications resolved in all (2 lost to follow up). Rate of complications significantly decreased between the first 50 treatments and the remainder (initial 82 % (95 % CI: 59-111 %) vs subsequent 29% (95 % CI: 16-49 %), p=0.0004).
Discussion
Overall, MRgFUS decreased total and viable tumor volume, with relief in pain and improvement in quality of life. The excellent soft tissue contrast and real-time thermometry of MRI allows conformal targeting and treatment monitoring. Patient treatment outcomes were hampered by challenges in obtaining insurance authorization, as many with residual or recurrent viable tumor may have benefited from additional treatment sessions; the addition in 2020 of ablation to the National Comprehensive Cancer Network (NCCN) guidelines as a primary or salvage option for desmoids may mitigate this challenge (18). Complications were associated in part with a learning curve, as well as with proximity of the tumor to skin and nerve. An active skin cooling device has been introduced to reduce the risk of burns (20).Conclusion
MRgFUS appears to be an effective treatment option for desmoid tumors, with a reasonable safety profile compared to the morbidity of other treatment options.Acknowledgements
No acknowledgement found.References
1. Middleton SB, Frayling IM, Phillips RK. Desmoids in familial adenomatous polyposis are monoclonal proliferations. Br J Cancer. 2000;82(4):827-32. Epub 2000/03/25. doi: 10.1054/bjoc.1999.1007. PubMed PMID: 10732754; PubMed Central PMCID: PMCPMC2374411.2. Alman BA, Pajerski ME, Diaz-Cano S, Corboy K, Wolfe HJ. Aggressive fibromatosis (desmoid tumor) is a monoclonal disorder. Diagn Mol Pathol. 1997;6(2):98-101. Epub 1997/04/01. doi: 10.1097/00019606-199704000-00005. PubMed PMID: 9098648.
3. Li M, Cordon-Cardo C, Gerald WL, Rosai J. Desmoid fibromatosis is a clonal process. Hum Pathol. 1996;27(9):939-43. Epub 1996/09/01. doi: 10.1016/s0046-8177(96)90221-x. PubMed PMID: 8816889.
4. Bonvalot S, Desai A, Coppola S, Le Pechoux C, Terrier P, Domont J, et al. The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol. 2012;23 Suppl 10:x158-66. Epub 2012/09/26. doi: 10.1093/annonc/mds298. PubMed PMID: 22987953.
5. De Bree E, Keus R, Melissas J, Tsiftsis D, van Coevorden F. Desmoid tumors: need for an individualized approach. Expert Rev Anticancer Ther. 2009;9(4):525-35. Epub 2009/04/21. doi: 10.1586/era.09.9. PubMed PMID: 19374605.
6. Merchant NB, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer. 1999;86(10):2045-52. Epub 1999/11/26. PubMed PMID: 10570430.
7. Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17(1):158-67. Epub 1999/08/24. doi: 10.1200/JCO.1999.17.1.158. PubMed PMID: 10458229.
8. Bishop AJ, Zarzour MA, Ratan R, Torres KE, Feig BW, Wang WL, et al. Long-Term Outcomes for Patients With Desmoid Fibromatosis Treated With Radiation Therapy: A 10-Year Update and Re-evaluation of the Role of Radiation Therapy for Younger Patients. Int J Radiat Oncol Biol Phys. 2019;103(5):1167-74. Epub 2018/12/16. doi: 10.1016/j.ijrobp.2018.12.012. PubMed PMID: 30552963.
9. Bates JE, Morris CG, Iovino NM, Rutenberg M, Zlotecki RA, Gibbs CP, et al. Radiation Therapy for Aggressive Fibromatosis: The Association Between Local Control and Age. Int J Radiat Oncol Biol Phys. 2018;100(4):997-1003. Epub 2018/02/28. doi: 10.1016/j.ijrobp.2017.12.259. PubMed PMID: 29485080.
10. Rutenberg MS, Indelicato DJ, Knapik JA, Lagmay JP, Morris C, Zlotecki RA, et al. External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer. 2011;57(3):435-42. Epub 2011/07/12. doi: 10.1002/pbc.22916. PubMed PMID: 21744472.
11. Mir O, Honore C, Chamseddine AN, Domont J, Dumont SN, Cavalcanti A, et al. Long-term Outcomes of Oral Vinorelbine in Advanced, Progressive Desmoid Fibromatosis and Influence of CTNNB1 Mutational Status. Clin Cancer Res. 2020;26(23):6277-83. Epub 2020/09/03. doi: 10.1158/1078-0432.CCR-20-1847. PubMed PMID: 32873570.
12. Azzarelli A, Gronchi A, Bertulli R, Tesoro JD, Baratti D, Pennacchioli E, et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer. 2001;92(5):1259-64. Epub 2001/09/26. doi: 10.1002/1097-0142(20010901)92:5<1259::aid-cncr1446>3.0.co;2-y. PubMed PMID: 11571741.
13. Skapek SX, Ferguson WS, Granowetter L, Devidas M, Perez-Atayde AR, Dehner LP, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol. 2007;25(5):501-6. Epub 2007/02/10. doi: 10.1200/JCO.2006.08.2966. PubMed PMID: 17290057.
14. Nishida Y, Tsukushi S, Shido Y, Wasa J, Ishiguro N, Yamada Y. Successful treatment with meloxicam, a cyclooxygenase-2 inhibitor, of patients with extra-abdominal desmoid tumors: a pilot study. J Clin Oncol. 2010;28(6):e107-9. Epub 2009/12/23. doi: 10.1200/JCO.2009.25.5950. PubMed PMID: 20026797.
15. Jo JC, Hong YS, Kim KP, Lee JL, Lee J, Park YS, et al. A prospective multicenter phase II study of sunitinib in patients with advanced aggressive fibromatosis. Invest New Drugs. 2014;32(2):369-76. Epub 2014/01/16. doi: 10.1007/s10637-013-0059-0. PubMed PMID: 24425345.
16. Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol. 2011;22(2):452-7. Epub 2010/07/14. doi: 10.1093/annonc/mdq341. PubMed PMID: 20622000.
17. Fiore M, Colombo C, Radaelli S, Callegaro D, Palassini E, Barisella M, et al. Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer. 2015;51(18):2800-7. Epub 2015/11/26. doi: 10.1016/j.ejca.2015.08.026. PubMed PMID: 26602014.
18. Molloy AP, Hutchinson B, O'Toole GC. Extra-abdominal desmoid tumours: a review of the literature. Sarcoma. 2012;2012:578052. Epub 2012/09/12. doi: 10.1155/2012/578052. PubMed PMID: 22966217; PubMed Central PMCID: PMCPMC3431123.
19. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-47. Epub 2008/12/23. doi: 10.1016/j.ejca.2008.10.026. PubMed PMID: 19097774.
20. Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72(2):288-306. Epub 2020/01/20. doi: 10.1016/j.jhep.2019.09.026. PubMed PMID: 31954493.
21. Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS, et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2017;28(10):1432-7 e3. Epub 2017/08/02. doi: 10.1016/j.jvir.2017.06.019. PubMed PMID: 28757285.
22. National Comprehensive Cancer Network. Clinical practice guidelines in oncology (NCCN guidelines) soft tissue sarcoma version 2. 2019. www.nccn.org. 2020.
23. Merrill R, Odeen H, Dillon C, Bitton R, Ghanouni P, Payne A. Design and evaluation of an open-source, conformable skin-cooling system for body magnetic resonance guided focused ultrasound treatments. Int J Hyperthermia. 2021;38(1):679-90. Epub 2021/04/27. doi: 10.1080/02656736.2021.1914872. PubMed PMID: 33899653.
Figures
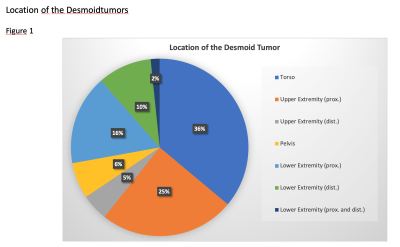
Location of the desmoid tumors
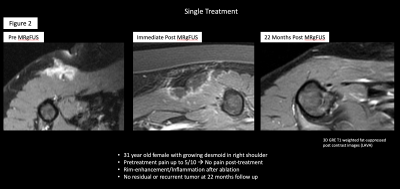
Single treatment of a desmoid tumor with eradication
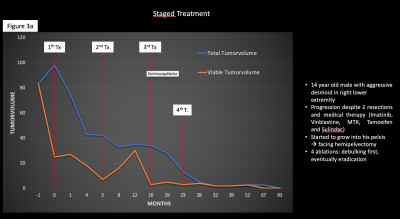
3a.: Patient with multiple treatments for highly aggressive desmoid tumor. The tumor was successfully debulked and eventually eradicated (patient history and tumor volume changes)
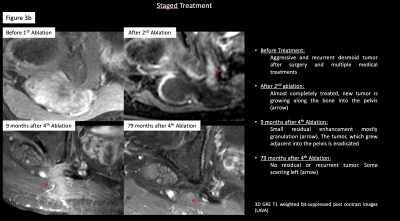
3b.: Patient with multiple treatments for highly aggressive desmoidtumor. The tumor was successfully debulked and eventually eradicated (Imaging)
Quality of Life (SF-36)
DOI: https://doi.org/10.58530/2022/2893