2890
Associations between DCE-MRI parameters and MVD, VEGF and HIF-1α in osteosarcoma based on MRI-histopathological comparison1Department of Radiology, The Third Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China, 2Department of Radiology, HangZhou Medical College Affiliated Lin'An People's Hospital, Hangzhou, Zhejiang Province, China, 3Clinical Science, Philips Healthcare, Chengdu, China, Chengdu, China
Synopsis
Tumor necrosis, angiogenesis and hypoxia are related to therapeutic effect, aggressiveness, metastasis and prognosis for osteosarcoma. In this study, DCE-MRI were matched ROI-to-ROI with histopathological tissues to explore the relationship between the perfusion parameters, including Ktrans, Kep, and Ve in each ROI and TNR, MVD, VEGF and HIF-1α in corresponding histopathological specimens, aiming to offer information about angiogenesis and hypoxia in the microenvironment of osteosarcoma timely and non-invasively. We found the perfusion parameters of DCE-MRI have a moderate sensitivity and specificity to evaluate MVD, VEGF and HIF-1α in the histopathological tissues of osteosarcoma, thereby helping microvessel status and hypoxia evaluating.
Objective
To investigate associations between the perfusion parameters of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and microvessel density (MVD), vascular endothelial growth factor (VEGF) and hypoxia inducible factor-1α (HIF-1α) in osteosarcoma based on MRI-histopathological comparison.Materials and Methods
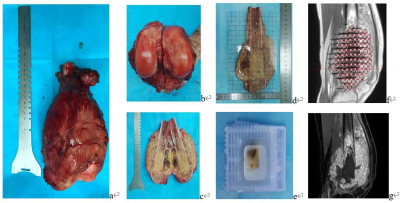
10 patients with osteosarcoma in the distal femur and proximal tibia were enrolled consecutively in a prospective study. After neoadjuvant chemotherapy, routine MRI and DCE-MRI were performed. After operation, 5 mm thick tissue slice was taken from the median sagittal plane of the gross specimen and divided into 1 cm × 1 cm tissue blocks. The tumor necrosis rate (TNR), MVD, VEGF and HIF-1α of each tissue block were detected. According to Huvos grading system, specimens were classified into responder group (TNR ≥ 90%) but excluded complete necrosis ones (TNR almost 100%) and non-responder group (TNR < 90%). On the median sagittal DCE-MRI images, the square regions of interest (ROI) of 1 cm × 1 cm were located where the specimens were taken and the perfusion parameters, including the volume transfer constant (Ktrans), the reflux rate constant (Kep), and the extravascular extracellular volume fraction (Ve) were measured. The relationship between perfusion parameters and MVD, VEGF, HIF-1α was analyzed by Poisson regression and logistic regression respectively, and receiver operating characteristic (ROC) curve was drawn to calculate the diagnostic threshold, sensitivity and specificity.Results
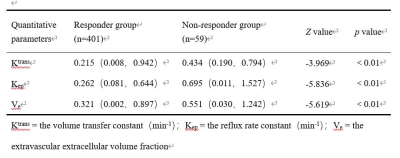
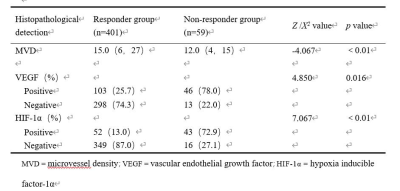
A total of 460 histopathological specimens were obtained in 10 patients with osteosarcoma, including 401 (87.2%) in responder group and 59 (12.8%) in non- responder group; 149 (32.4%) were VEGF positive and 311 (67.6%) were negative; 95 (20.7%) were HIF-1α positive and 365 (79.3%) were negative. Ktrans, Kep, Ve, MVD and the positive rate of VEGF in the responder group were higher than those in the non- responder group, while the positive rate of HIF-1α was lower than that in the non- responder group (P < 0.05). Ktrans, Kep and Ve were correlated with MVD, and the regression coefficients were 0.652, 0.525 and -0.418, respectively (P < 0.01). The threshold, area under the curve (AUC), sensitivity and specificity of Ktrans for diagnosis of VEGF and Kep for HIF-1α were 0.115 min−1, 0.672, 65.1%, 66.1% and 0.663 min−1, 0.745, 52.4%, 89.8%, respectively.Discussion
DCE-MRI has been widely adapted in malignant tumors with the advantages of real-time and repeatably monitoring, which can effectively reflect the tumor perfusion and capillary permeability, and evaluate it non-invasively 1, 2, 3, 4, 5, 6, 7.TNR is the pathological "gold standard" for evaluating the efficacy of NAC in osteosarcoma 8, 9, 10. If the proliferation of tumor is active, the tumor cells are densely arranged, the extracellular space will be small, the number of immature microvessels and capillary permeability will increase, so Ktrans and Kep values will increase, and Ve will decreases. On the contrary, tumor necrosis and extracellular space increases, and microvessel density decreases after NAC, so the Ktrans and Kep values decrease, and Ve value increases accordingly. The results of present study are consistent with previous studies 4, 5, 6, 7. herefore, DCE-MRI parameters could be used as an early predictor of event-free survival and total survival days in patients with good response to NAC. Tumor angiogenesis and microvessels distribution are related to tumor proliferation, which can be evaluated with MVD count in lesions. In this study, we observed the similar performance in the tissue blocks of osteosarcoma and we also found that in soft tissue region with high MVD count, most of the microvessels were mature and the distortion rate was low, while in the central tumorous osteogenesis area, the MVD count was low with poor differentiation of microvessel. VEGF is one of the most important initiation inducing factors in tumor angiogenesis pathway and the increased expression of HIF-1α plays a key role in the adaptation of tumor to hypoxia 11. DCE-MRI is a useful, non-invasive imaging technique for evaluation of VEGF and HIF-1α expression, especially Ktrans and Kep could assess VEGF and HIF-1α expression in our study, which is supported by the previous investigations.
In present study, the image-histopathological comparison method was used according to the method of TNR of osteosarcoma. Each ROI of the lesion in DCE-MRI was exactly matched with histopathological specimen of the tumor section, which may be more accurate to reflect the relationship between the quantitative parameters and and histopathological information of each specimen.
There are also several limitations in this prospective study. Firstly, this study is a single center study with a small number of enrolled cases, and the distribution of pathological specimens are uneven, which may affect the results to some extent. Therefore, larger prospective studies are warranted to validate the clinical value of DCE-MRI quantitative parameters analysis. Secondly, the heterogeneity of osteosarcoma is obvious, and the histopathological changes after neoadjuvant chemotherapy are more complex 1, 8, 10.
Conclusion
In conclusion, the present study found that the DCE-MRI perfusion parameters had a moderate sensitivity and specificity to evaluate MVD, VEGF and HIF-1α in the histopathological tissues of osteosarcoma, thereby helping microvessel status and hypoxia evaluating, but its implementation, MR parameter setting and data analysis have not been unified between different MR scanners and institutions, which needs further studies.Acknowledgements
We thank all the patients who participated in this study. We thank Dr. Xinxin Fan from the Second Affiliated Hospital of Kunming Medical University for useful discussions on data analysis. This work was supported by Yunnan Fundamental Research Projects (grant No. 2019FE001-079 and No. 2019FE001-240).References
1. Gaustad JV, Anette H, Catherine S, et al. DCE-MRI of tumor hypoxia and hypoxia-associated aggressiveness. Cancers (Basel). 2020; 12(7): 1979.
2. Isabelle C, Franck V, Vincent C, et al. The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells. 2020; 9(4): 976-1001.
3. Lilienthal I, Herold N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: a review of current and future strategies. Int J Mol Sci. 2020; 21(18): 6885.
4. Sahoo P, Gupta RK, Gupta PRK, et al. Diagnostic accuracy of automatic normalization of CBV in glioma grading using T1-weighted DCE-MRI. Magn Reson Imaging. 2017; 44: 32-37.
5. Kang SR, Kim HW, Kim HS. Evaluating the relationship between dynamic contrast-enhanced MRI (DCE-MRI) parameters and pathological characteristics in breast cancer. J Magn Reson Imaging. 2020; 52(2): 1360-1373.
6. Tönnes C, Janssen S, Golla AK, et al. Deterministic arterial input function selection in DCE-MRI for automation of quantitative perfusion calculation of colorectal cancer. Magn Reson Imaging. 2021; 75: 116-123.
7. Li XW, Wang QM, Dou YP, et al. Soft tissue sarcoma: can dynamic contrast-enhanced (DCE) MRI be used to predict the histological grade. Skeletal Radiol 2020; 49(11): 1829-1838.
8. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010; 21(7): 320-325.
9. Maya K, Michele WT, Mark JS, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014; 14(11): 722-735.
10. NIU XH. Interpretation of clinical practice guidelines for management of typical osteosarcoma. Chin Clin Oncol. 2012; 17(10): 934-945.
Figures