2889
Evaluation of Deep-Learning Reconstructed High-Resolution 3D Cervical Spine MRI to Improve Foraminal Stenosis Evaluation1Department of Radiology and Imaging, Hospital for Special Surgery, New York, NY, United States, 2GE Healthcare, Chicago, IL, United States, 3Biostatistics Core, Research Administration, Hospital for Special Surgery, New York, NY, United States
Synopsis
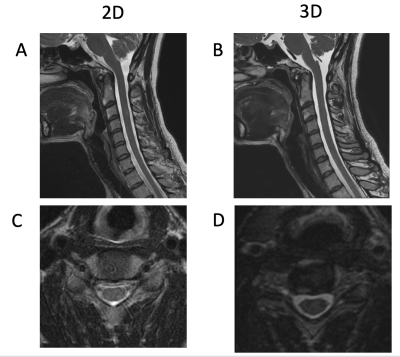
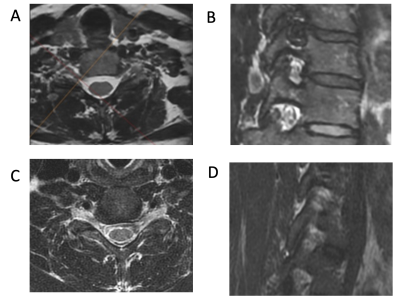
Isotropic 3D MRI, with the addition of deep-learning based reconstruction algorithms (DLRecon), facilitates faster acquisition times and multiplanar reformatting. We compared interobserver agreement for cervical spine neural foraminal (NF) stenosis assessment of 3D T2-weighted fast spin echo (T2w-FSE) MR images with DLRecon to those of standard-of-care (SOC) 2D T2w-FSE images. We demonstrated that inter-observer agreement was high for both 2D and 3D sequences in the assessment of NF stenosis, but agreement was more consistent between readers at each level for the 3D acquisition. 3D DLRecon images were also more frequently free of motion, when compared to corresponding 2D sequences.
Introduction
Three-dimensional (3D) acquisitions provide multiple benefits over standard-of-care (SOC) 2D MRI due to isotropic voxel acquisition that facilitates overall shorter acquisition times due to the ability to create multiplanar reformations from a single data set, and for the cervical spine (c-spine), decreased CSF flow artifacts. The ability to reformat image data beyond conventional planes can prove especially useful when assessing obliquely-oriented structures such as the cervical neural foramina (NF). However, results of 3D vs 2D MRI comparisons of the c-spine have been mixed, with some studies demonstrating improved visualization of certain anatomic structures on 3D sequences, but no significant differences in visualization of pathology1–4; this was perhaps due to relatively lower SNR or lower in-plane spatial resolution of 3D compared to 2D acquisitions. The recent application of deep-learning based reconstruction (DLRecon) algorithms to 3D MRI, however, has provided higher SNR and image sharpness5. Commercially available 2D DLRecon algorithms have now been incorporated into clinical protocols, but 3D DLRecon is not yet commercially available or well-studied.For this study, we hypothesized that improved image quality from 3D DLRecon, combined with the added benefit of 3D multiplanar reformatting, would result in improved interobserver agreement for c-spine NF stenosis as compared to 2D SOC acquisitions.Methods
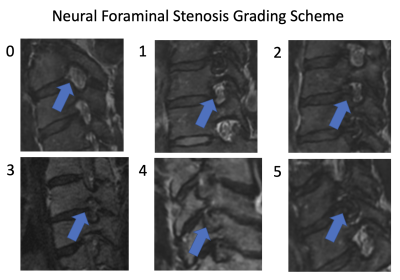
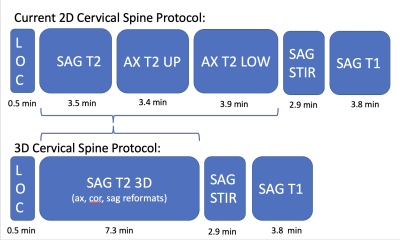
In this IRB-approved study, images of 20 patients, who underwent routine c-spine MRI with SOC 2D T2w-FSE and isotropic 3D T2w-FSE sequences, the latter processed with 3D DLRecon, were retrospectively reviewed by three readers: a musculoskeletal radiologist (5 years’ experience), a neuroradiologist (9 years’ experience) and a musculoskeletal radiology fellow. Scans were acquired on a clinical 3T MRI unit (Signa Premier, GE Healthcare) with a 21-channel head-neck coil. Evaluated sequences included a 3D T2w-FSE (FOV=24cm, acquired 0.7mm-isotropic, TR/TE = 1502/100 ms), two 2D T2w-FSE axial sequences (upper and lower batches) (FOV=16cm, 0.5x0.8mmx3mm, TR/TE = 4500/120 ms), and a 2D T2w-FSE sagittal sequence (FOV=22cm, 0.6x0.8x3mm, TR/TE = 5000/110 ms). 2D SOC and 3D DLRecon images were then randomized for qualitative evaluation by the readers for the degree of motion artifact using a 3 point scale (0=none, 1=mild, 2=moderate, 3=severe), as well as for NF and central stenosis using a predefined 4-6 point grading scale tailored to each variable of interest, (previously validated in the published literature6). Statistical analysis was performed utilizing weighted Fleiss’s kappa (k) to determine inter-rater agreement for each variable.Results
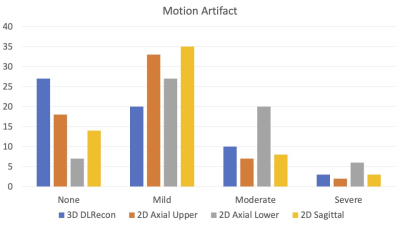
Inter-rater agreement for degree of NF stenosis was substantial for both 2D and 3D T2w-FSE sequences (k = 0.756 and 0.812, respectively). While estimates of agreement for degree of NF stenosis were similar between 2D and 3D sequences overall, agreement was more consistent at each level for 3D images (sd=0.11) than 2D images (sd=0.15). For the degree of central stenosis at each motion segment, inter-rater agreement was high for both 2D SOC and 3D DLRecon sequences (k = 0.850 and 0.832, respectively). There was less inter-rater agreement for the degree of motion, with composite k values of 0.551 and 0.479 for 2D and 3D sequences, respectively. However, DLRecon sequences were more often scored as devoid of motion by the three readers (27/60), compared with SOC 2D upper axial (18/60), 2D lower axial (7/60), and 2D sagittal images (14/60). For the 2D SOC T2w-FSE sequences, mean acquisition time for sagittal, upper axial and lower axial sequences was 3.5 minutes, 3.4 minutes and 3.9 minutes, respectively, with mean total scan time of 10.8 minutes. In comparison, the 3D T2w-FSE sequence had a mean scan time of 7.3 minutes.Discussion
Our study demonstrated that use of a deep-learning based reconstruction algorithm (DLRecon) for 3D c-spine MRI provided improved or equivalent agreement of NF or central stenosis assessment, respectively, as compared to SOC 2D imaging. The high interobserver agreement for NF stenosis, with improved agreement on 3D sequences, is likely a reflection of improved image quality, as well as the ability for readers to utilize multiplanar reformations to assess the neural foramina via optimal (orthogonal) anatomical planes. The consistently high interobserver agreement on both 2D SOC T2w-FSE and 3D T2w-FSE DLRecon images for central stenosis likely reflects the ability of readers to perform more objective measurements of canal diameter. Our preliminary results also suggest that motion artifacts may be reduced in 3D compared to 2D acquisitions, similar to that in the lumbar spine7, and may be due to shorter scan time or differences in view-ordering methods used in 3D vs. 2D interleaved acquisitions8. We intend to complete assessment of a total of 41 subjects to confirm these results. Our study was limited by the lack of evaluation of 3D SOC MRI sequences (without DLRecon), and 2D DLRecon sequences. However, comparisons of DLRecon had previously been performed for both 2D5 and for 3D, in particular in the lumbar spine where improvements in image quality from applying DLRecon have been recognized9. Additionally, while our assessment was limited to T2w-FSE sequences, benefits of 3D DLRecon may extend to fat-suppressed and T1-weighted sequences as well.Conclusion
3D T2w-FSE imaging of the cervical spine, when combined with a deep learning-based reconstruction algorithm, provides high inter-reader agreement of neural foraminal stenosis assessment that is more consistent relative to conventional 2D sequences, despite shorter total acquisition time.Acknowledgements
We would like to acknowledge institutional research funding from GE Healthcare, and thank GE scientists Maggie Fung, Marc Lebel and Shiv Kaushik for their technical assistance.References
1. Chokshi FH, Sadigh G, Carpenter W, Allen JW. Diagnostic Quality of 3D T2-SPACE Compared with T2-FSE in the Evaluation of Cervical Spine MRI Anatomy. AJNR Am J Neuroradiol. 2017;38(4):846-850. doi:10.3174/ajnr.A5080
2. Fu MC, Buerba RA, Neway WE, et al. Three-Dimensional Isotropic MRI of the Cervical Spine: A Diagnostic Comparison With Conventional MRI. Clin Spine Surg. 2016;29(2):66-71. doi:10.1097/BSD.0b013e3182a355e5
3. Kwon JW, Yoon YC, Choi SH. Three-dimensional isotropic T2-weighted cervical MRI at 3T: comparison with two-dimensional T2-weighted sequences. Clin Radiol. 2012;67(2):106-113. doi:10.1016/j.crad.2011.06.011
4. Xiao L, Siu CWJ, Yeung K, Leung A, Yuen MK, Wong YC. MRI of the cervical spine with 3D gradient echo sequence at 3 T: initial experience. Clin Radiol. 2015;70(9):926-931. doi:10.1016/j.crad.2015.05.012
5. Zochowski KC, Tan ET, Argentieri EC, et al. Improvement of peripheral nerve visualization using a deep learning-based MR reconstruction algorithm. Magn Reson Imaging. 2021;85:186-192. doi:10.1016/j.mri.2021.10.038
6. Argentieri EC, Koff MF, Breighner RE, Endo Y, Shah PH, Sneag DB. Diagnostic Accuracy of Zero-Echo Time MRI for the Evaluation of Cervical Neural Foraminal Stenosis. Spine. 2018;43(13):928-933. doi:10.1097/BRS.0000000000002462
7. Koontz NA, Wiggins RH, Mills MK, et al. Less Is More: Efficacy of Rapid 3D-T2 SPACE in ED Patients with Acute Atypical Low Back Pain. Acad Radiol. 2017;24(8):988-994. doi:10.1016/j.acra.2017.02.011
8. Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55(5):1030-1037. doi:10.1002/mrm.20863
9. Sun S, Tan ET, Carrino J, Mintz D, Sahr M, Endo Y, et al. Evaluation of Deep-Learning Reconstructed High-Resolution 3D Lumbar Spine MRI to Improve Image Quality. Proc ISMRM 2021.:pp805.
10. Uetani H, Nakaura T, Kitajima M, Yamashita Y, Hamasaki T, Tateishi M, et al. A preliminary study of deep learning-based reconstruction specialized for denoising in high-frequency domain: usefulness in high-resolution three-dimensional magnetic resonance cisternography of the cerebellopontine angle. Neuroradiology. 2021;63(1):63–71.
11. Kashiwagi N, Tanaka H, Yamashita Y, Takahashi H, Kassai Y, Fujiwara M, et al. Applicability of deep learning-based reconstruction trained by brain and knee 3T MRI to lumbar 1.5T MRI. Acta Radiologica Open. 2021;10(6):20584601211023940.
12. Ogawa R, Kido T, Nakamura M, Nozaki A, Lebel RM, Mochizuki T, et al. Reconstruction of cardiovascular black-blood T2-weighted image by deep learning algorithm: A comparison with intensity filter. Acta Radiologica Open. 2021;10(9):20584601211044780.
Figures