2886
Comparison of AI-assisted Compressed Sensing (ACS) and Conventional Two-Dimensional Sequences on Lumbar Spine Imaging
He Sui1, Yu Gong2, Yunfei Zhang3, Yongming Dai3, and Zhanhao Mo1
1China-Japan Union Hospital of Jilin University, Changchun, China, 2Linyi People’s Hospital, Linyi, China, 3Central Research Institute, United Imaging Healthcare, Shanghai, China
1China-Japan Union Hospital of Jilin University, Changchun, China, 2Linyi People’s Hospital, Linyi, China, 3Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
MRI is widely used in the diagnosis of spinal diseases, but due to the limitation of long scanning time, it is often difficult for patients to tolerate. This study aims at exploring whether the ACS (AI-assisted Compressed Sensing) accelerated sequences can assure similar image quality and diagnostic accuracy of lumbar spine diseases compared to 2D conventional sequences. Results suggested that 2D ACS sequence has become a well-anticipated technique for routine examination of lumbar spine and lumbar spine lesions diagnosis, while ensuring high image quality and reduced scan time effectively than traditional 2D sequences in MRI.
Introduction:
Magnetic resonance imaging (MRI) is widely used to assess spinal diseases due to its safety, non-ionizing nature and predominant soft tissue resolution1. However, the acquisition time of MRI is too long, and it is difficult for patients with back pain to keep still during the examination, which would lead to motion artifacts and affect the imaging quality2. Therefore, the need of accelerated magnetic resonance scanning is imminent. In addition to classic acceleration technology such as GRAPPA3, SPIRiT4 and SAKE5, whose imaging speed cannot meet clinical needs currently. There are some updated data acquisition strategies emerged aiming to accelerate MRI data acquisition speed and reduce scan time, including simultaneous multi-slice or multi-band imaging, compressed sensing (CS) MRI, synthetic MRI and MR fingerprinting6-8. These methods are mostly used in 3D imaging and functional MRI, notably in spectroscopy, dynamic cardiovascular applications and angiography. Nonetheless, 2D scanning is still the mainstream scanning mode for spinal MR imaging where these methods still present certain limitations. The imaging qualities of these strategies are unsatisfactory in clinical practice, which is easy to cause diagnostic bias. With the rapid development of artificial intelligence (AI) in recent years, the wide application of convolutional neural network (CNN) based on traditional accelerating technologies has revealed its powerful potential in accelerated MRI9-12. In view of aforementioned issues, we intend to evaluate and compare the image quality and diagnostic accuracy of ACS sequences, as an acceleration method for MRI combining PI (Parallel Imaging), HF (Half Fourier), CS (Compressed Sensing) and AI Network and 2D conventional sequences for lumbar spine.Methods:
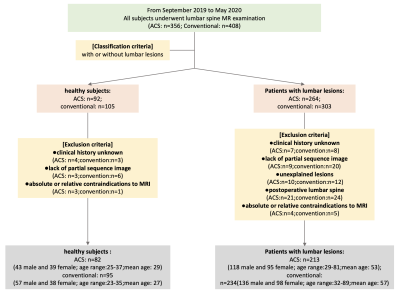
We collected data from 82 healthy subjects and 213 patients who used 2D ACS accelerated sequences to examine lumbar spine while 95 healthy subjects and 234 patients used 2D conventional sequences (Fig.1). The MRI imaging sequences included axial T2WI, sagittal T2WI, T1WI, and T2-fs sequences were collected with a 3.0 scanner (uMR 780, United Imaging Healthcare, Shanghai, China). All obtained images of these subjects were analyzed with RadiAnt DICOM Viewer (5.5.1) software in the light of calculating image quality- factors such as signal to noise ratio (SNR) and contrast to noise ratio (CNR) for selected regions of interest. The lumbar image quality, artifacts and visibility of lesion structure were assessed by two radiologists independently using five-point scale and three-point scale. Differences between the evaluation values above were tested for statistical significance by the Wilcoxon signed-ranks test. Inter-observer agreements of image quality between two radiologists were measured using Cohen’s kappa correlation coefficient.Results:
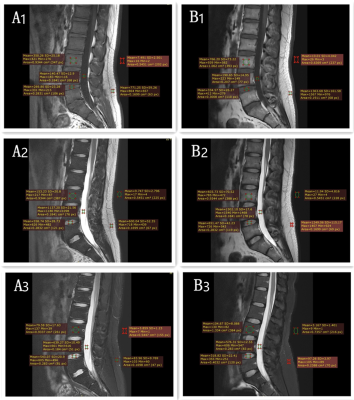
Similar image quality was achieved with 2D conventional, and ACS accelerated sequences in both healthy subjects and patients. There were no significant differences in quantitative SNR and CNR measurements for every study group (p>0.05) (Fig.2). No significant differences were found between the qualitative scores within reviewing radiologists (p>0.05), and there was significant difference in artifacts between two sequences(p<0.05). Moreover, Inter-observer agreement between two radiologists in scoring image quality was substantial consistently for ACS accelerated sequences and conventional sequences (k =0.622-0.986).Discussion:
The current study implemented ACS acceleration methods to reduce scan times while maintaining the image quality, which is relative to conventional acquisition using consistent scan parameters of coverage, voxel-size and bandwidth. The ACS technique, which combines four acceleration techniques, has improved the acceleration capacity compared with other acceleration techniques, especially in 2D MR imaging. Also, its reliability has been improved compared with pure AI-based method. It is worth mentioning that the ACS accelerated sequences has applied to the clinical multi-site imaging and reached satisfactory results. As a result, ACS should be a valuable option for patients with back pain to avoid scan time limitations while as well as to provide high-quality images for clinical diagnosis.Conclusion:
Comparing with 2D conventional sequence, ACS accelerated sequences allow for faster lumbar spine imaging with the same imaging quality as traditional sequences. In addition, ACS accelerated sequences could present similar diagnostic accuracy in evaluating lumbar spine lesions.Acknowledgements
NoneReferences
1. G OEH, J NJ, M VAC, Z GA, G MHM. Mr imaging of the menisci and cruciate ligaments: A systematic review. Radiology 2003;2262. Bitar R, Leung G, Perng R, Tadros S, Moody Alan R, Sarrazin J et al. Mr pulse sequences: What every radiologist wants to know but is afraid to ask. Radiographics : a review publication of the Radiological Society of North America, Inc 2006;26
3. Mark AG, Peter MJ, Robin MH, Mathias N, Vladimir J, Jianmin W et al. Generalized autocalibrating partially parallel acquisitions (grappa). Magnetic Resonance in Medicine 2002;47
4. Martin U, Peng L, Mark JM, Patrick V, Michael E, John MP et al. Espirit—an eigenvalue approach to autocalibrating parallel mri: Where sense meets grappa. Magnetic Resonance in Medicine 2014;71
5. Peter MJ, Mark AG, Robert RE, Daniel K. Sodickson %+ Department of Radiology BIDMCHMS, Department of Medicine CDBIDMCHMS. Auto-smash: A self-calibrating technique for smash imaging. Magma: Magnetic Resonance Materials in Physics, Biology, and Medicine 1998;7
6. Steen M, Essa Y, Cheryl AO, Edward A, John S, Noam H et al. Multiband multislice ge‐epi at 7 tesla, with 16‐fold acceleration using partial parallel imaging with application to high spatial and temporal whole‐brain fmri. Magnetic Resonance in Medicine 2010;63
7. Donoho DL. Compressed sensing. IEEE Transactions on Information Theory 2006;52:1289-1306
8. Park S, Kwack Kyu-Sung %+ 1 Department of Radiology AUSoMSSK, Musculoskeletal Imaging Laboratory AUMCSSK, Department of Radiology AUSoMSSK, Musculoskeletal Imaging Laboratory AUMCSSK. Initial experience with synthetic mri of the knee at 3t: Comparison with conventional t<sub>1</sub> weighted imaging and t<sub>2</sub> mapping. The British journal of radiology 2018;91 %W CNKI
9. Yang G, Yu S, Dong H, Slabaugh G, Dragotti Pier L, Ye X et al. Dagan: Deep de-aliasing generative adversarial networks for fast compressed sensing mri reconstruction. IEEE transactions on medical imaging 2018;37
10. Wang S, Su Z, Ying L, Peng X, Zhu S, Liang F et al. Accelerating magnetic resonance imaging via deep learning. Proceedings. IEEE International Symposium on Biomedical Imaging 2016;2016
11. Schlemper J, Caballero J, Hajnal Joseph V, Price Anthony N, Rueckert D. A deep cascade of convolutional neural networks for dynamic mr image reconstruction. IEEE transactions on medical imaging 2018;37
12. Yang Y, Sun J, Li H, Xu Z. Admm-csnet: A deep learning approach for image compressive sensing. IEEE transactions on pattern analysis and machine intelligence 2020;42
DOI: https://doi.org/10.58530/2022/2886