2884
Abnormal Cerebral Microstructures in Amyotrophic Lateral Sclerosis: a Diffusion Kurtosis Imaging Study1Fujian Medical University Union Hospital, Fuzhou, China, 2Radiology, Fujian Medical University Union Hospital, Fuzhou, China
Synopsis
Our study aim to investigate cerebral microstructural changes in Amyotrophic lateral sclerosis (ALS) using diffusion kurtosis imaging (DKI) for the first time. ALS patients showed mean kurtosis metrics (MK) reductions in several gray/white matter areas. The spatial distribution of the regions with reduced radial kurtosis(RK) was similar to those with decreased MK. No significant axial kurtosis(AK) difference was found between groups. The correlation analysis revealed significant associations between DKI metrics and clinical assessments. Additionally, several white matter regions showed between-group differences in conventional diffusion metrics; but the spatial extent was smaller than that with reduced DKI metrics.
Introduction
In adults, the most frequent motor neuron disease is amyotrophic lateral sclerosis (ALS), which features progressive muscular weakness1. Therefore, it is important to fully elucidate the brain structural and functional alterations related to this disease, since this can provide new insights into its neuropathology and can help identify novel treatment targets and/or potential biomarkers for assessing disease severity and therapy2. Neuroimaging findings show that the gray matter (GM) and white matter (WM) are atrophied and WM microstructures are impaired in ALS, which is strongly supported by relevant neuropathological reports that demonstrate neuron loss, axonal degeneration, and consequent reactive gliosis in ALS3;4;5. In the current study we investigated the microstructural alterations related to ALS throughout whole brain using diffusional kurtosis imaging (DKI). The advantages of DKI over the conventional diffusion tensor imaging (DTI) technique are mainly two-fold. First, DKI takes into account the fundamentally non-Gaussian property of water diffusion in real and complex brain tissues, leading to better neural tissue characterization6;7. DKI has been suggested as a promising approach to offer a more comprehensive and sensitive detection of brain tissue microstructural alterations in several pathological conditions, such as mild cognitive impairment and Alzheimer’s disease, Parkinson’s disease, epilepsy, and traumatic brain injure8. Second, DKI allows assessment of microstructural characterization in both GM and WM simultaneously, which facilitates whole-brain exploration of microstructural changes in various neurological disorders9.Methods
Subjects: Eighteen ALS patients and 20 healthy controls.Field Strength/Sequence: DKI images were obtained by a spin-echo echo-planar imaging sequence on a 3T MRI scanner, with three b-values (0, 1000, and 2000 s/mm2) and 64 diffusion encoding directions.
Assessment: The revised ALS Functional Rating Scale (ALSFRS-R) was administered to assess disease severity, and the symptom duration and disease progression rate were also recorded. Voxel-based analysis was applied to examine the alteration of DKI metrics (ie, mean kurtosis metrics [MK], axial kurtosis [AK], and radial kurtosis [RK]) and the conventional diffusion metrics (ie, fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity).
Statistical Tests: Student’s t-test, chi-square test, and Pearson correlation analysis.
Results
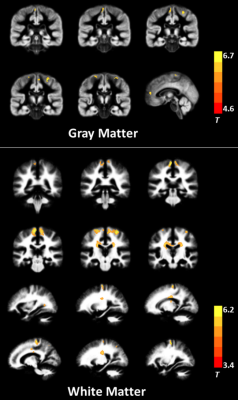
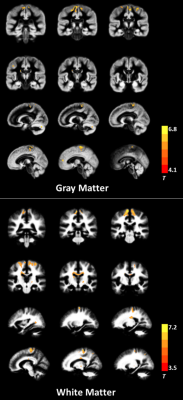
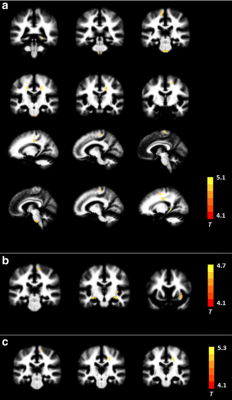
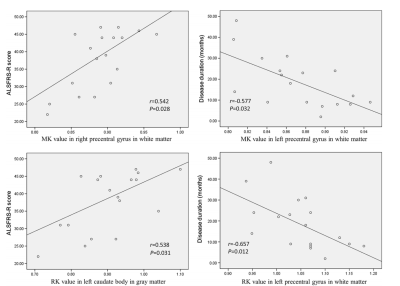
ALS patients showed MK reductions in gray matter areas, including the bilateral precentral gyrus, bilateral paracentral lobule, and left anterior cingulate gyrus; they also showed decreased MK values in white matter (WM) in the bilateral precentral gyrus, bilateral corona radiata, bilateral middle corpus callosum, left occipital lobe, and right superior parietal lobule. The spatial distribution of the regions with reduced RK was similar to those with decreased MK. No significant AK difference was found between groups. The correlation analysis revealed significant associations between DKI metrics and clinical assessments such as ALSFRS-R score and disease duration. Additionally, several WM regions showed between-group differences in conventional dif- fusion metrics; but the spatial extent was smaller than that with reduced DKI metrics.Conclusion
The reduction in DKI metrics indicates decreased microstructural complexity in ALS, involving both motor-related areas and extramotor regions. DKI metrics can serve as potential biomarkers for assessing disease severity.Acknowledgements
The National Natural Science Foundation of China (No. 82071900) and Fujian Province Joint Funds for the Innovation of Science and Technology (No. 2019Y9067) supported this study.References
1. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med 2001;344:1688–1700.
2. Chio A, Pagani M, Agosta F, Calvo A, Cistaro A, Filippi M. Neuroimaging in amyotrophic lateral sclerosis: Insights into structural and functional changes. Lancet Neurol 2014;13:1228–1240.
3. Alrafiah AR. From mouse models to human disease: An approach for amyotrophic lateral sclerosis. In Vivo 2018;32:983–998.
4. Chang JL, Lomen-Hoerth C, Murphy J, et al. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 2005;65:75–80.
5. Garcia-Santibanez R, Burford M, Bucelli RC. Hereditary motor neuropathies and amyotrophic lateral sclerosis: A molecular and clinical update. Curr Neurol Neurosci Rep 2018;18:93.
6. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005;53: 1432–1440.
7. Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage 2009;45:386–392.
8. Marrale M, Collura G, Brai M, et al. Physics, techniques and review of neuroradiological applications of diffusion kurtosis imaging (DKI). Clin Neuroradiol 2016;26:391–403.
9. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005;53: 1432–1440.
Figures