2883
Comprehensive brainstem imaging using MT, QSM and DTI for atypical parkinsonism diagnosis1Department of Radiology and Nuclear medicine, Erasmus MC, Rotterdam, Netherlands, 2Department of Neurology, Erasmus MC, Rotterdam, Netherlands, 3Department of Imaging Physics, TU Delft, Delft, Netherlands
Synopsis
Differentiating atypical parkinsonism (AP) from idiopathic Parkinson’s disease (PD) remains a challenge in clinical practice. Based on a recent review on differentiating atypical parkinsonisms using MRI, we developed a dedicated MRI protocol including magnetization transfer (MT), quantitative susceptibility mapping (QSM) and diffusion tensor imaging (DTI) for improving diagnosis. Using pilot data from a healthy volunteer, we show how the assessment of important structures (substantia nigra and locus coeruleus) can be improved using a comprehensive combination of MRI techniques.
Introduction
Differentiating atypical parkinsonism (AP) from idiopathic Parkinson’s disease (PD) remains a challenge in clinical practice. It is estimated that a quarter of atypical parkinsonisms are misdiagnosed as Parkinson’s disease1.A recent review2 found three MRI techniques to have sensitivies and specificites of 80% and above to differentiate diagnosed PD and AP patients, using specific midbrain structures, namely:
- Quantitative susceptibility mapping (QSM) to quantify iron accumulation in midbrain structures such as the substantia nigra (SN) substructures, pars compacta (SNpc) and pars reticulata (SNpr), as well as the red and subthalamic nucleus3,4.
- Quantifying brainstem atrophy5,6 as well as cerebellar peduncle and third ventricle atrophy7 on high-resolution T1-weighted MPRAGE images.
- Fractional anisotropy (FA) to quantify demyelination of white matter tracts8,9 in the brainstem structures such as the superior and middle cerebral peduncle (SCP and MCP).
Having all four techniques could give the necessary information required to make a differential diagnosis of suspected patients. Previous studies10,12,13 use volumetric or area-derived metrics from small structures (substantia nigra, locus coeruleus, cerebral peduncles) as biomarkers, but structure delineation and understanding confounding factors can be challenging when using only one contrast. Previous studies have combined neuromelanine with iron imaging for similar purposes10,14,15.
The disappereance of the LC is an relevant biomarker11, however imaging and locating the LC is challenging. Brainstem DTI could offer an additional landmark.
In this work, we demonstrate initial in-vivo results from a healthy volunteer using a dedicated atypical parkinsonism protocol, to explore how acquiring all four contrasts could aid interpretation and diagnosis when used in patients.
Methods
The dedicated Parkinsonism protocol was developed on a 3T GE Signa Premier MRI scanner equipped with a 48 channel head-coil.For one volunteer (female, 18yo) we acquired a sagittal T1-SPGR (Flip angle 12o, matrix size 240x240x176, voxel size 1mm isotropic, acq time 4:41), an axial MT-weighted (flip angle 40o, 2D-GRE, flip angle 40o, matrix size 512x320x20, voxel size 0.4x0.7x1.5mm, vendor MT pulse with offset 1200Hz, TE 8.1ms, TR 240ms, NEX 3, acq time 12 minutes), a 3D multi-echo gradient echo (TE1=13ms, ...,TE8=37.6ms,echo-spacing 3.5ms, matrix 320x226x82, voxel size 0.86x0.x86x1mm) and a DTI scan (singleshot EPI, matrix 128x128x50, voxel size 1.75x1.75x2mm, TE 77.0ms, TR 2869ms, SMS2, phase acceleration 2, 10 interleaved volumes with b=0 and 64 directions with b=1000).
QSM images were produced using the MEDI toolbox16,17. DTI-derived FA maps were produced using FSL dtifit18, after susceptibility19 and eddy current correction20. All images were co-registered to the T1-weighted image using MITK25.
Results
Figure 1 shows the 4 imaging techniques used in this work.Figure 2 zooms in around the substantia nigra. MT-weighted imaging shows the neuromelanine-rich substantia nigra pars compacta (SNpc) as a hyperintense area (Fig. 2AB) and the MT+QSM overlay shows the iron-rich neighbouring substructures of the substantia nigra, the pars reticulata (SNpr) (Fig. 2CD). The overlap in QSM and NM signal could be due both iron and NM being present in both substructures, and signal changes in the overlap was found as a useful PD biomarker13. Adjacent to these are white matter tracts from the cerebral peduncle (Fig. 2EF), which are highly anisotropic as shown clearly as an area with high FA in an FA map. Parkinson patients are expected to lose neuromelanine and accumulate iron in the substantia nigra, and having multiple contrasts could improve area delineation.
Figure 3 shows the three contrasts around the locus coeruleus, two thin rod-shaped structures in the pons rich in neuromelanine (Fig. 3AB) which due to their small size are difficult to image. Figures 3CD shows the LC posterior to two longitudinal WM tracts, that are visible using FA, which probably represent the medial or dorsal longitudinal fasciculus (MLF or DLF), or a combination of both. This relationship between the LC and MLF could be useful when locating the LC in parkinsonism patients for whom, due to neuromelanine loss, the LC becomes less visible with disease progression11. Reduced FA in the Superior Cerebral Peduncle (SCP, annotated in purple) is a potential biomarker for AP8,9. Figures 3EF shows elevated susceptibility spots at the same location, which could be due to iron content from the LC21.
Discussion and conclusion
We imaged structures related to atypical parkinsonism by integrating the combined information obtained from 4 pertinent MRI techniques. Visualising diffusion metrics, iron and neuromelanine concentration relies on different forms of neurodegeneration, however interpreting confounding effects (e.g. iron and DTI22,23) is a subject of future research.Many recent studies on differentiating parkinsonism use combined QSM-and-neuromelanine10,14,15 images for ROI-based image analysis. DTI-metrics have been used in the same way24. We show that having all three could aid localisation and interpretation.
Our pilot result was based on one healthy volunteer, future work will investigate its merit in patient populations and other known brainstem structures identified as biomarkers2.
In conclusion, a dedicated parkinsonism imaging protocol could improve the delineation of anatomical structures, improving ROI-based methods for the differential diagnosis of atypical parkinsonisms.
Acknowledgements
This project was funded by Erasmus MC – TU Delft Convergence for health and technology.
This research was financially supported by ParkinsonNL.
References
1. Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002;125(Pt 4):861-870.2. Chougar L, Pyatigorskaya N, Degos B, Grabli D, Lehericy S. The Role of Magnetic Resonance Imaging for the Diagnosis of Atypical Parkinsonism. Front Neurol 2020;11:665.
3. Sjostrom H, Granberg T, Westman E, Svenningsson P. Quantitative susceptibility mapping differentiates between parkinsonian disorders. Parkinsonism Relat Disord 2017;44:51-57.
4. Mazzucchi S, Frosini D, Costagli M, Del Prete E, Donatelli G, Cecchi P, Migaleddu G, Bonuccelli U, Ceravolo R, Cosottini M. Quantitative susceptibility mapping in atypical Parkinsonisms. Neuroimage Clin 2019;24:101999.
5. Mangesius S, Hussl A, Krismer F, Mahlknecht P, Reiter E, Tagwercher S, Djamshidian A, Schocke M, Esterhammer R, Wenning G, Muller C, Scherfler C, Gizewski ER, Poewe W, Seppi K. MR planimetry in neurodegenerative parkinsonism yields high diagnostic accuracy for PSP. Parkinsonism Relat Disord 2018;46:47-55.
6. Quattrone A, Nicoletti G, Messina D, Fera F, Condino F, Pugliese P, Lanza P, Barone P, Morgante L, Zappia M, Aguglia U, Gallo O. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 2008;246(1):214-221.
7. Quattrone A, Morelli M, Nigro S, Quattrone A, Vescio B, Arabia G, Nicoletti G, Nistico R, Salsone M, Novellino F, Barbagallo G, Le Piane E, Pugliese P, Bosco D, Vaccaro MG, Chiriaco C, Sabatini U, Vescio V, Stana C, Rocca F, Gulla D, Caracciolo M. A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson's disease. Parkinsonism Relat Disord 2018;54:3-8.
8. Planetta PJ, Ofori E, Pasternak O, Burciu RG, Shukla P, DeSimone JC, Okun MS, McFarland NR, Vaillancourt DE. Free-water imaging in Parkinson's disease and atypical parkinsonism. Brain 2016;139(Pt 2):495-508.
9. Du G, Lewis MM, Kanekar S, Sterling NW, He L, Kong L, Li R, Huang X. Combined Diffusion Tensor Imaging and Apparent Transverse Relaxation Rate Differentiate Parkinson Disease and Atypical Parkinsonism. AJNR Am J Neuroradiol 2017;38(5):966-972.
10. Takahashi H, Watanabe Y, Tanaka H, Mihara M, Mochizuki H, Liu T, Wang Y, Tomiyama N. Quantifying changes in nigrosomes using quantitative susceptibility mapping and neuromelanin imaging for the diagnosis of early-stage Parkinson’s disease. The British Journal of Radiology 2018;91(1086):20180037.
11. Ohtsuka C, Sasaki M, Konno K, Kato K, Takahashi J, Yamashita F, Terayama Y. Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Parkinsonism Relat Disord 2014;20(7):755-760.
12. Hartono S. Susceptibility Map-Weighted Imaging and Neuromelanin-Sensitive MRI in Parkinson’s Disease. Proc. Intl. Soc. Mag. Reson. Med. 28. (1511) 2020.
13. Rua C, O’Callaghan C, Ye R, Hezemans FH, Passamonti L, Jones PS, Williams GB, Rodgers CT, Rowe JB. Substantia nigra ferric overload and neuromelanin loss in Parkinson’s disease measured with 7T MRI. medRxiv 2021:2021.2004.2013.21255416.
14. Jin L, Wang J, Wang C, Lian D, Zhou Y, Zhang Y, Lv M, Li Y, Huang Z, Cheng X, Fei G, Liu K, Zeng M, Zhong C. Combined Visualization of Nigrosome-1 and Neuromelanin in the Substantia Nigra Using 3T MRI for the Differential Diagnosis of Essential Tremor and de novo Parkinson's Disease. Front Neurol 2019;10:100.
15. Reimao S, Ferreira S, Nunes RG, Pita Lobo P, Neutel D, Abreu D, Goncalves N, Campos J, Ferreira JJ. Magnetic resonance correlation of iron content with neuromelanin in the substantia nigra of early-stage Parkinson's disease. Eur J Neurol 2016;23(2):368-374.
16. Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, Tsiouris AJ, Wisnieff C, Spincemaille P, Prince MR, Wang Y. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012;59(3):2560-2568.
17. Liu T, Khalidov I, de Rochefort L, Spincemaille P, Liu J, Tsiouris AJ, Wang Y. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed 2011;24(9):1129-1136.
18. Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003;50(5):1077-1088.
19. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23 Suppl 1:S208-219.
20. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016;125:1063-1078.
21. Zucca FA, Bellei C, Giannelli S, Terreni MR, Gallorini M, Rizzio E, Pezzoli G, Albertini A, Zecca L. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. J Neural Transm (Vienna) 2006;113(6):757-767.
22. Zhang J, Tao R, Liu C, Wu W, Zhang Y, Cui J, Wang J. Possible effects of iron deposition on the measurement of DTI metrics in deep gray matter nuclei: an in vitro and in vivo study. Neurosci Lett 2013;551:47-52.
23. Schneider TM, Ma J, Wagner P, Behl N, Nagel AM, Ladd ME, Heiland S, Bendszus M, Straub S. Multiparametric MRI for Characterization of the Basal Ganglia and the Midbrain. Front Neurosci 2021;15:661504.
24. Langley J, Huddleston DE, Merritt M, Chen X, McMurray R, Silver M, Factor SA, Hu X. Diffusion tensor imaging of the substantia nigra in Parkinson's disease revisited. Hum Brain Mapp 2016;37(7):2547-2556.
25. Medical Imaging Interaction Toolkit - https://github.com/MITK/MITK. Available from: https://github.com/MITK/MITK.
Figures
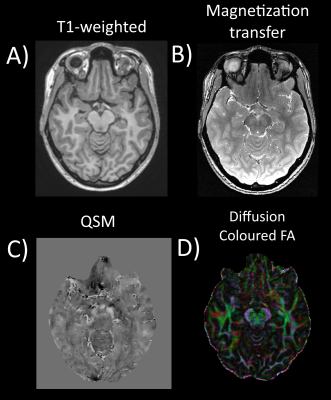
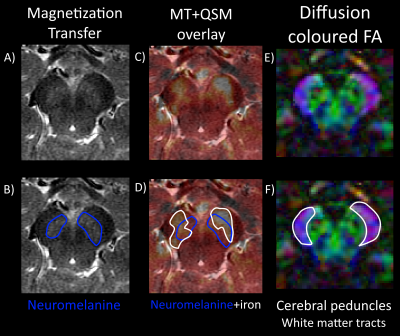
Figure 2: Axial zoom in on the substantia nigra (SN) showing the topological order of the structure. A) Neuromelanine MRI shows neuromelanine outlined in B) present in the SN pars compacta (SNpc), an SN substructure known to be rich in which alters its concentration for AP patients. C) A neuromelanine-MRI with QSM in a partial red overlay reveals iron outlined in white in D) present in the SN pars reticulata (SNpr) which increases in PD patients. E) A DTI-derived FA-map shows high anisotropy where low susceptibility was found, clearly delineating the F) cerebral peduncles from the SN.
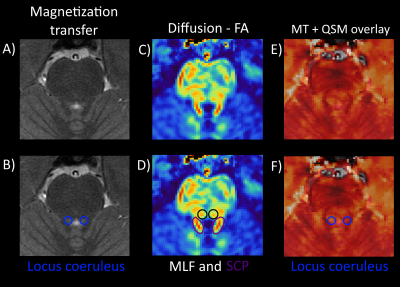
Figure 3: Zoom around the locus coeruleus (LC). A) MT-weighted MRI shows the LC annotated in B) as small hyperintense spots from neuromelanine. C) FA image, 1 slice lower than the other images, shows the SCP (D) in purple) and medial longitudinal fasciculus (MLF) located anterior to the LC, annotated in D) with black circles. This relationship could be useful when locating the LC in patients with neuromelanine loss making the LC less visible. E) MT with QSM overlay show small hyperintense regions at the same location, which could be due to iron in the LC21 F).