2881
Mind the Myelin: Investigating the therapeutic impact of exercise on white matter damage in a rodent model of Fetal Alcohol Spectrum Disorders1Psychological & Brain Sciences, University of Delaware, NEWARK, DE, United States
Synopsis
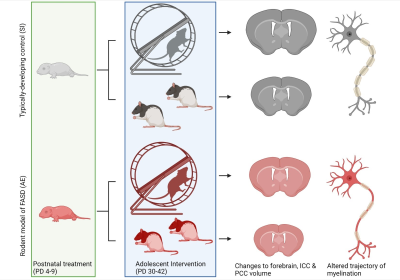
Fetal Alcohol Spectrum Disorders (FASD) is an umbrella term used to identify individuals with a history of prenatal alcohol exposure which results in a spectrum of diagnostic disorders. 1 in 20 infants born in the U.S. has been diagnosed with an FASD, creating a major public health crisis. Deficits in corpus callosum myelination resulting from prenatal alcohol exposure have been correlated with impairments to perceptual learning and executive function in adolescents diagnosed with FASD. This study investigates the therapeutic potential of an exercise intervention to ameliorate alcohol-induced damage to corpus callosum myelination in a rodent model of FASD.
Introduction
Fetal Alcohol Spectrum Disorders (FASD) resulting from prenatal alcohol exposure have a high prevalence around the globe, with 1 in 20 live births in the U.S. being affected annually1. Adolescents affected by FASD exhibit reductions in myelination to the corpus callosum, a major commissural white matter tract, and this structural anomaly is correlated with several cognitive and behavioral deficits including impairments to perceptual reasoning and executive function2.CNS myelination is imperative for optimizing neuronal signaling. A unique feature of myelination in the mammalian brain is that it begins during the brain growth spurt and continues to be modified through adolescence to refine brain circuitry that will support cognitive processing in adulthood3. As a result, the trajectory of myelination is easily influenced by early-life and adolescent experiences such as exercise, social interaction, and learning4. Thus, we hypothesized that exposure to a myelination-stimulating exercise intervention in adolescence might mitigate the impact of prenatal alcohol exposure on corpus callosum development. Previously, the Klintsova lab has demonstrated that increased aerobic activity in adolescence or adulthood restores neuroplasticity in a well-established rat model of FASD5.
Methods
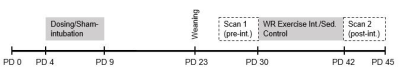
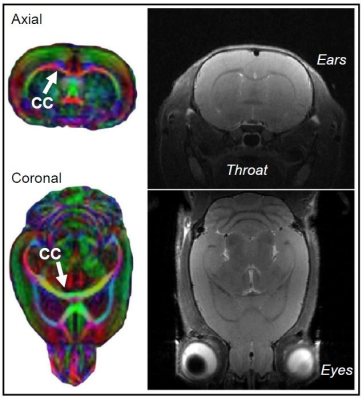
This study employs in vivo diffusion tensor imaging (DTI) scanning to investigate the effects of: 1) neonatal alcohol exposure and 2) an adolescent exercise intervention on corpus callosum myelination in a rodent model of FASD. Male and female Long-Evans rat pups were alcohol-exposed via intragastric intubation (5.25 g/kg/day) on postnatal days (PD) 4-9, targeting the rodent brain growth spurt. Sham-intubated controls received no liquid during intubation. From PD 30-42 in adolescence, half of all rats had free access to a running wheel for aerobic intervention. DTI scans were acquired twice longitudinally (on PD 30 and 42) in all rats using a 9.4T Bruker Biospec scanner to assess alterations to corpus callosum myelination noninvasively. Corpus callosum was divided into three sub-regions for analysis: the interhemispheric region (ICC) and the left and right cortically-projecting region (LPCC and RPCC). Voxel size is 250µm x 250µm x 500µm and scans from the entire brain were captured resulting in a total scan time of half an hour per session.Results
Analysis using mixed repeated measures ANOVAs (within-subjects variable: weight/day; between-subjects variables: postnatal treatment and intervention groups; covariate: sex) indicate that fractional anisotropy increases in ICC (F1, 74 = 38.2, p < .001) and left/right PCC (F1, 75 = 17.6, p < .001; F1, 75 = 22.8, p < .001) across development, as expected. Further, analysis with two and three-way ANOVAs (postnatal treatment x sex; postnatal treatment x intervention exposure with sex as a covariate, respectively) for data collected on PD 30 and 42 show that fractional anisotropy values are lower in alcohol-exposed rats compared to control rats at both time points (p < .001), indicating that water is moving more freely within corpus callosum in adolescent rats with previous alcohol exposure.Analysis using mixed repeated measures ANOVAs (within-subjects variable: weight/day; between-subjects variables: postnatal treatment and intervention groups; covariate: sex) indicates that radial diffusivity decreases in ICC (F1, 74 = 84.7, p < .001) and left/right PCC (F1, 75 = 17.5, p < .001; F1, 75 = 26.0, p < .001) across adolescence, as expected with increasing myelination. However, analyses using two and three-way ANOVAs (postnatal treatment x sex; postnatal treatment x intervention exposure with sex as a covariate, respectively) for data collected on PD 30 or 42 show that radial diffusivity values remain higher in alcohol-exposed compared to control rats in all corpus callosum sub-regions (p < .001). These findings suggest that alcohol exposure during the brain growth spurt reduces corpus callosum myelination in adolescence.
Discussion
As expected, we show that age has an opposite but significant effect on the fractional anisotropy and radial diffusivity across adolescence in all sub-regions of corpus callosum, indicating that the white matter is developing in all animals in this study. There was no significant interaction with exercise intervention. However, cross-sectional analysis at each time point confirms that fractional anisotropy and radial diffusivity values in all corpus callosum sub-regions are reduced in AE compared to control rats. Importantly, when we compare values of anisotropy and diffusivity in the AE brain on PD 42 to those from the control brain on PD 30, we do not see any significant differences. Taken together, we interpret these data to show that AE during the brain growth alters the trajectory of myelination in corpus callosum leading to an observed hypomyelination in adolescence when compared to controls. This is in line with numerous clinical observations of white matter development in teenagers with FASD.Conclusion
Future research is needed to investigate if AE leads to a persistent loss of myelination in CC in adulthood. More importantly, to comprehensively examine the immediate and long-term effects of exercise on myelination, future studies will include histological analysis of brain tissue samples collected from these rats. Finally, increasing the length of the study in an effort to examine the lasting effects of exercise on myelination in the adult brain is warranted.Acknowledgements
The authors would like to acknowledge our funding sources: NIH/NIAAA R01AA027269 and UD COBRE: MRI Pilot Funding 2P20GM103653-06 awarded to Dr. Anna Klintsova.References
1May, P. A., Keaster, C., Bozeman, R., Goodover, J., Blankenship, J., Kalberg, W. O., et al. Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug Alcohol Depend. 2015; 155, 118–127.
2Wozniak, J. R., and Muetzel, R. L. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol. Rev. 2011; 21, 133–147.
3Semple, B. D., Blomgren, K., Gimlin, K., Ferriero, D. M., and Noble-Haeusslein, L. J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013; 106–107, 1–16.
4Tomlinson, L., Leiton, C. V., and Colognato, H. Behavioral experiences as drivers of oligodendrocyte lineage dynamics and myelin plasticity. Neuropharmacology 2016; 110, 548–562.
5Gursky, Z. H., and Klintsova, A. Y. Wheel Running and Environmental Complexity as a Therapeutic Intervention in an Animal Model of FASD. J. Vis. Exp, 2017; 54947.
Figures