2873
Respiration robust universal pulses in the human heart at 7T for shallow and deep breathing1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2German Cancer Research Center (DKFZ), Medical Physics in Radiology, Heidelberg, Germany, 3University of Minnesota, Center for Magnetic Resonance Research, Minneapolis, MN, United States
Synopsis
This study demonstrates the design and application of a new class of universal pulses (UP) designed for a library of 22 non-respiration resolved (NRR) B1+-maps acquired in-vivo during shallow breathing (SB) and 30 respiration resolved (RR) B1+-maps acquired during deep breathing (DB). The proposed UP was tested on 9 NRR SB (test-cases-SB) and 15 RR DB B1+-maps (test-cases-DB) and was applied experimentally in five volunteers (test-cases-DB) along with tailored pulses in multiple gradient echo measurements. Compared to tailored pulses, UP resulted in a slightly overall decrease of the FA homogeneity but similar image quality across all test-cases.
Introduction
MRI in the human body at ultra-high fields (>7T) is typically limited by motion and strong inter-subject variations of the spatial flip-angle (FA) patterns caused by varying body shapes and dimensions. Tailored parallel transmission (pTx)1 for each subject based on subject-specific B1+-maps2,3 or pre-computed universal pulses (UPs)4,5 independent of the subject have been successfully used to reduce spatial FA variations. For deep breathing (DB), however, a recent study showed severe B1+ changes between deep inhale and exhale resulting in FA changes for tailored pTx pulses during respiration.6 This can be avoided by generating tailored respiration-robust (RRob) pulses using respiration resolved (RR) B1+-maps from multiple respiration states.6 Here, we design UPs to robustly achieve homogeneous FA distributions throughout the heart for SB and DB. The proposed respiration-robust UP was pre-computed on 22 non-respiration resolved (NRR) SB and 30 RR-DB B1+-maps and was successfully validated experimentally at 7T in five unseen volunteers for SB and DB.Methods
MRI was performed on a Siemens Magnetom 7T scanner using an 8-channel transmit array and a whole-body gradient system according to an approved IRB protocol after written informed consent in a total of 46 healthy volunteers in four different groups: i) library-SB: 14M/8F, 21-66years, BMI=20-28kg/m2, ii) test-cases-SB: 4M/5F, 25-56years, BMI=19-35kg/m2, iii) library-DB: 4M/6F, 24-56years, BMI=21-28kg/m2 and iv) test-cases-DB: 4M/1F, 22-40years, BMI=20-25kg/m2. The scans were performed with a 32-element body array (MRI-Tools, Berlin) driven in 8Tx/32Rx mode. Safety limits and coil placements were identical to the previous works.2,3,5Relative 3D NRR and RR thoracic B1+-maps were acquired under free-breathing in 3min25s for SB (256 radial-phase encoded7 (RPE) lines, groups i-iv and in 6min50s for DB (512 RPE lines, groups iii-iv).3 Common parameters were: nominal FA=20°, TE/TR=2.02/40ms, FOV=250x312x312mm3, resolution=4x4x4mm3. The SB data was reconstructed NRR whereas the DB data was reconstructed RR into five respiration states using self-navigation.
Three UPs were designed based on three different libraries: i) UP-SB: library-SB, ii) UP-DB: library-DB and iii) UP-SBDB: library-SB and library-DB. Furthermore, tailored-NRR or respiration robust (RRob) tailored-RRob pulses were designed for each subject for SB and DB, respectively, and a default shim setting (optimized by the manufacturer based on EM-models) was applied. The pulses were designed for a trait-off between RF power and FA root-mean-squared-error8 and their performance was analysed using the coefficient-of-variation (CV) in the manually selected heart volumes. The source code and the B1+-maps can be downloaded from https://github.com/chaigner/UP_body and https://github.com/chaigner/tailored-RRob.
3D gradient-echo (GRE) scans with parameters fitting to the B1+-scans have been acquired in all five volunteers of group test-cases-DB to validate the tailored pulses and the precomputed UP-SBDB during SB and DB. The SB GRE data was reconstructed NRR and the DB GRE data was reconstructed RR for five respiration states similar to the B1+-maps.
Results and Discussion
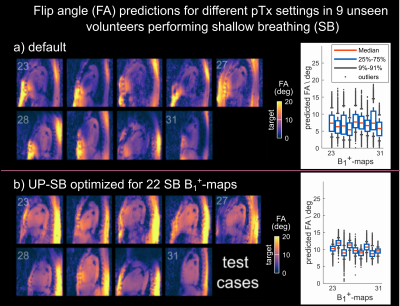
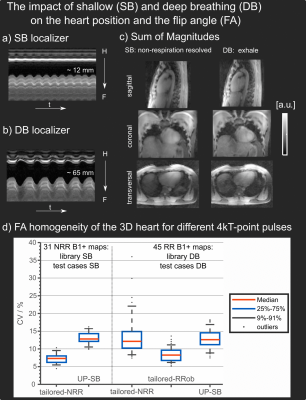
Figure 1 illustrates the spatial FA predictions in nine unseen volunteers of test-cases-SB for a sagittal slice using the default setting (a) and UP-SB (b) to achieve a nominal FA of 10 degrees in the 3D heart ROI. Excellent FA homogeneity with a median CV of 12.8% (compared to a median CV of 44% using the default setting) is achieved despite large inter-subject BMI variations.Animated Figure 2 illustrates the breathing amplitude for SB (a) and DB (b), the impact of respiratory motion on the sum-of-magnitude GRE images (c) and on the performance of different pTx pulses. Tailored-NRR and UP-SB achieved median CVs (CVmedian) of 7% and 12.8% across library-SB and test-cases-SB. Slightly larger CV variations are observed when applying tailored-NRR to library-DB and test-cases-DB with CVmedian=12.1% and outliers up to 36% whereas UP-SB resulted in a similar median CV of 12.6% as for test-cases-SB. The best performance in library-DB/test-cases-DB yielded tailored-RRob with CVmedian=8.6%.
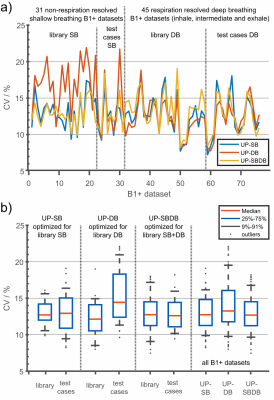
Figure 3 shows the CV of all 76 B1+-maps (31 NRR-SB, 45 RR-DB) using three excitation settings: UP-SB, UP-DB and UP-SBDB. UP-SB and UP-SBDB achieve more homogeneous FAs than UP-DB for the unseen test-cases with a CVmedian=12.9% and 12.8% vs. 14.4%. Moreover, UP-DB resulted in outliers up to 22.1% which might reflect an insufficiently large database of just 10 subjects. In summary, UP-SBDB performed best, however, UP-SB performed surprisingly well despite not using any RR B1+-maps in the pulse design.
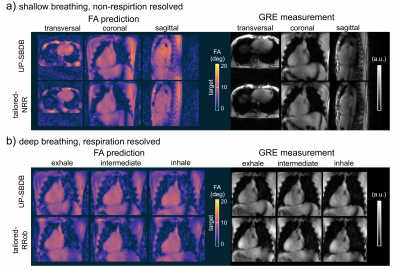
Figure 4 shows 3D FA predictions for SB (a) and DB (b) and NRR and RR 3D GRE images acquired with tailored-NRR, tailored-RRob and UP-SBDB. Qualitatively, a close match between FA predictions and the 3D GRE images were observed and no substantial differences (e.g., signal dropouts) between tailored-NRR, tailored-RRob and UP-SBDB were found.
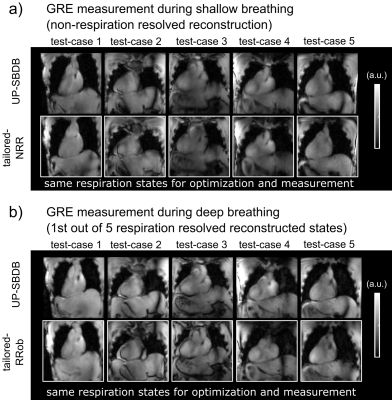
Animated Figure 5 shows one coronal slice across the heart of the acquired 3D GRE volume for SB (a) and DB (b) for all test-cases-DB using tailored-NRR, tailored-RRob and pre-computed UP-SBDB. Qualitatively, a close match between tailored and UP-SBDB was found in the 3D GRE images demonstrating the feasibility of calibration-free pTx in the human heart in the case of SB but also in the case of DB.
Conclusion
This study demonstrates in-vivo that UP-SB and UP-SBDB can be robust for respiratory motion and that UPs are therefore highly suitable for calibration-free 3D heart FA homogenization at 7T for time-critical situations without the need for a lengthy (SB~10-15min and DB~20-30min) calibration process.Acknowledgements
We gratefully acknowledge funding from the German Research Foundation SCHM 2677/2-1 and GRK2260, BIOQIC.References
1) Padormo, F., Beqiri, A., Hajnal, J. V., and Malik, S. J. (2016), Parallel transmission for ultrahigh‐field imaging. NMR in Biomed., 29: 1145– 1161. doi: 10.1002/nbm.3313
2) Aigner, C.S., Dietrich, S., and Schmitter, S. (2020), Three-dimensional static and dynamic parallel transmission of the human heart at 7T, NMR in Biomed., e4450. doi:10.1002/nbm.4450
3) Dietrich, S., Aigner, C.S., and Schmitter, S. (2021), 3D Free-breathing Multi-channel absolute B1+ Mapping in the Human Body at 7T, Magn. Reson. Med., doi:10.1002/mrm.28602
4) Gras, V., Vignaud, A., Amadon, A., Le Bihan, D., and Boulant, N. (2017), Universal pulses: A new concept for calibration‐free parallel transmission. Magn. Reson. Med., 77: 635-643. doi:10.1002/mrm.26148
5) Aigner, C.S, Dietrich, S., Schaeffter, T., and Schmitter, S. (2021) Calibration-free pTx of the human heart at 7T via 3D universal pulses. Magn Reson Med., 00: 1– 15. https://doi.org/10.1002/mrm.28952
6) Aigner, C.S, Dietrich, S., Schaeffter, T., and Schmitter, S Respiration induced B1+ changes and its compensation via respiration robust 3D kT point pulses in 7T body imaging. Proc. 29th Annu. Meet. ISMRM, virtual: 2021, p. 3947.
7) Prieto, C., Uribe, S., Razavi, R., Atkinson, D., and Schaeffter, T., (2010) 3D undersampled golden‐radial phase encoding for DCE‐MRA using inherently regularized iterative SENSE. Magn. Reson. Med., 64, pp. 514-526, doi:10.1002/mrm.22446
8) Cao, Z., Yan, X. and Grissom, W.A. (2016), Array‐compressed parallel transmit pulse design. Magn. Reson. Med., 76, pp. 1158-1169., doi:10.1002/mrm.26020
Figures