2872
Calibration-free parallel transmission for improved CSF mobility imaging at 7T1German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 2Radiology, Leiden University Medical Center, Leiden, Netherlands, 3University of Paris-Saclay, CEA, CNRS, BAOBAB, NeuroSpin, Gif sur Yvette, France, 4Siemens Healthcare SAS, Saint-Denis, France, 5Department of Physics and Astronomy, University of Bonn, Bonn, Germany
Synopsis
Better understanding of how the brain cleans itself is of high importance as its failure has been linked to various neurodegenerative diseases. A first step towards elucidating CSF-mediated clearance mechanisms is the measurement of CSF motion in the human brain. In this work, we present an imaging sequence for CSF mobility measurements employing calibration-free parallel transmission. During the imaging session, no pulse calculations or complex B1 shimming procedures are necessary, making this approach feasible for large scale patient studies.
Purpose
In the last years, UHF (7T) imaging has become an increasingly important modality for neuroscience applications. Namely, it was recently shown that UHF enables high resolution imaging of cerebrospinal fluid (CSF)-mobility in perivascular spaces of the human brain [1]. This is of high interest, as CSF has been suggested as the main carrier of waste products [2] from the brain, but the exact mechanisms still remain strongly debated [3,4]. Studying brain clearance mechanisms is therefore of particular importance since failure of waste-drainage is linked to pileup of toxic proteins leading to neurodegenerative diseases. Better understanding of these mechanisms is therefore urgent, as it would provide crucial new avenues not only to elucidate the pathophysiology of these diseases, but also towards new targets for efficient drugs to slow or stop disease progression. However, when currently using the classic RF transmission mode (circularly polarized), B1 and B0 inhomogeneities induce signal dropouts and/or insufficient suppression of unwanted tissue components. To overcome the aforementioned limitations, we propose a high field optimized CSF imaging technique employing universal parallel transmit pulses [5] that require no time consuming B1 shimming or pulse calculation procedures during the imaging session, making this a promising approach for patient studies at UHF.Methods
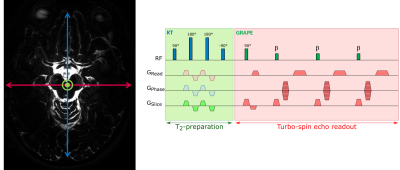
Experiments were performed on a whole-body investigational 7T scanner (Siemens Healthineers, Erlangen, Germany). Data were acquired using a head array coil with 32 receive and 8 transmit channels (Nova Medical, Wilmington, USA) and a modified TSE sequence with universal GRAPE pulses for excitation and refocusing [6] to mitigate B0 and B1 inhomogeneities. A long echo time was used to suppress tissue signal (TE=491 ms, TR=5000 ms, TSE-factor 146, refocusing FA=35deg). To encode CSF mobility (and to increase CSF selectivity even further), a modified parallel transmission T2-prep-module [7] (TEprep=43 ms) with velocity-encoding gradients of 0.6 cm/s was included. A schematic sequence diagram is shown in Figure 1. Seven sets of images were acquired to reconstruct a DTI-like tensor. Total scan time per venc was 3:18 min. Intra-subject images were registered using Elastix [8] and DTI postprocessing [9] was performed using Matlab to compute the principal CSF-flow direction.Results
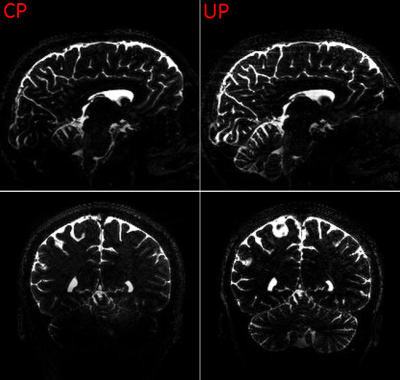
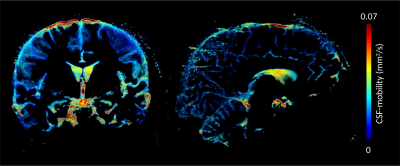
Figure 2 shows a comparison between images from a standard CP mode and full pTX acquisition. The standard acquisition results in signal dropouts in the temporal lobes and the cerebellum. In contrast, applying parallel transmission mitigates B0 and B1 field inhomogeneities and removes dropouts over the whole brain volume (Figure 2 right). As a consequence, CSF-mobility can be quantified in all regions of the brain (Figure 3).Discussion/Conclusion
In this work, we present an optimized MR sequence for CSF mobility imaging at UHF. The calibration-less parallel transmit approach preserves the strongly T2-weighted image contrast over the whole FoV. Visual quality of the resulting CSF mobility maps is clearly improved. However, mobility appears to be approximately two times larger than in previous work [10]. The source of this discrepancy is not quite clear to us, yet, and requires further investigation. In future work, we will try to increase the resolution while maintaining a reasonable scan time by employing sparse undersampling in combination with compressed sensing, similar to a previous study [10]. The final goal is to measure CSF-mobility in perivascular spaces in Alzheimer’s disease patient cohorts with high SNR and homogeneous contrast over the entire brain.Acknowledgements
No acknowledgement found.References
[1] Hirschler L. et al.: Proceedings ISMRM 2019, 0746.
[2] Wardlaw J. M. et al.: Nat. Rev. Neurol. 2020, doi:10.1038/s41582-020-0312-z.
[3] Dolgin E.: Nat. Biotechnol 2020, doi:10.1038/s41587-020-0443-1.
[4] Mestre H. et al.: Trends Neurosci 2020 xx, 1–9.
[5] Gras et al. Magn Reson Med. 2017;77,635-642
[6] van Damme et al.:MRM 2020;00:1–16
[7] Gras et al.: MRM 2019;81:3202–3208
[8] Klein et al.: IEEE Trans. Med. Imaging 2010;29:196–205
[9] Kroon, D.-J.: 2008 [online]: DTI and Fiber Tracking. Available at: https://www.mathworks.com/matlabcentral/fileexchange/21130-dti-and-fiber-tracking
[10] Hirschler L. et al.: Proceedings ISMRM 2020, 0643.
Figures