2868
Whole-liver flip angle shimming at 7T using eight-channel parallel transmission kt-points pulses with FPE-DREAM B1+ mapping
Bobby Runderkamp1, Wietske van der Zwaag2, Thomas Roos2, Gustav Strijkers3, Matthan Caan3, and Aart Nederveen1
1Radiology & Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands, 2Spinoza Center for Neuroimaging, Amsterdam, Netherlands, 3Biomedical Engineering and Physics, Amsterdam UMC, Amsterdam, Netherlands
1Radiology & Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands, 2Spinoza Center for Neuroimaging, Amsterdam, Netherlands, 3Biomedical Engineering and Physics, Amsterdam UMC, Amsterdam, Netherlands
Synopsis
Liver MRI could benefit from the increased SNR at 7T but suffers from severe flip angle inhomogeneity. In this work, eight-channel parallel transmission is used for flip angle shimming, comparing circularly polarized transmission and phase shimming with a kt-points pulse. Fourier phase-encoded DREAM is used for artifact-free B1+ mapping. With kt-points, the nominal flip angle of 8˚ could be achieved with homogeneous signal over the entire liver, while phase shimming only delivers a flip angle of approximately 4˚ with less homogeneity. Future work is directed towards use of universal slab-selective kt-points pulses to make 7T liver MRI clinically feasible.
Introduction
Liver MRI could benefit from the increased SNR at 7T. However, the B1+ wavelength decreases to abdominal dimensions at this field strength, causing flip angle (FA) inhomogeneity when using simple RF pulses in circularly polarized (CP) transmission. In this work, we use multi-channel parallel transmission for whole-liver FA homogenization comparing kt-points1 with phase shimming. This necessitates artifact-free B1+ mapping, which was done using Fourier phase-encoded (FPE) DREAM2,3.Methods
Eight healthy volunteers were scanned with a 7T Philips Achieva scanner (Philips, Best, The Netherlands) using an eight-channel Tx/Rx fractionated dipole antenna body array4 (MRCoils, The Netherlands, Figure 1c). For FPE-DREAM B1+ mapping, multiple B1+ measurements (‘modes’) are acquired with all channels emitting with continuously varying phase between modes according to $$$\exp\left(\frac{2\pi i\left(m-1\right)c}{M}\right)$$$, where m and c are the mode and channel number respectively and M is the total number of modes. In case M is higher than the number of channels, a weighting function is used to suppress unreliable measurements at low SNR from the overdetermined inverse problem3. After acquiring all modes, single-channel B1+ maps were calculated on-scanner which, together with a 2nd order B0-shimmed 3D-GRE, served as input for phase shimming and kt-points pulse calculation. To find the minimally necessary number of modes, a 35-year old male with a relatively high BMI of 28.4 was scanned with 8, 10, 13 and 16 modes. Figure 2a shows the eight-channel B1+ magnitudes and phases calculated using 13 modes and p=0.25, q=20 in the weighting function (eq. 9)3. Figure 2b shows single-channel B1+ magnitude maps calculated from these acquisitions. Based on these results, 13 modes were determined sufficient. Using those values, FA shimming was performed on seven volunteers (BMI 18.4-23.3, weight 50-78kg, age 26-34 years, 3 males). In volunteer 1-5, 3D-GRE scans were acquired using CP (45˚ phase increments between channels), phase shimming (except for volunteer 1) and kt-points. Additionally, intra- and inter-session kt-points repetitions were performed in four volunteers. The shimming volume-of-interest (VOI) encompassed the whole liver (see exemplary VOI in Figure 3, volunteer 5, kt-points). For phase shimming, the eight transmit-channel phase offsets were individually adapted to minimize the RF standard-deviation/(mean)2 over the VOI, using the MRCodeTool toolbox (MRCode, The Netherlands). Kt-points pulses were calculated with five subpulses, max. blip strength = 33 mT/m, max. blip slew rate = 166 mT/m/ms and a maximum excitation k-space extent of 1/6 cm-1. Channel RF amplitudes and phases and the 3D excitation k-space trajectory were calculated using an interleaved greedy-local optimization algorithm5 in a home-built MRCodeTool extension6 (Figure 1b). The kt-points pulses were non-selective, requiring large FOVs to cover the entire transmission volume of the antennae. All scans were acquired in a single breath-hold, as were all separate FPE-DREAM modes. Other scan settings are listed in Figure 1a. The scans were reconstructed offline using BART7 and MRecon (Gyrotools, Zurich, Switzerland) in MATLAB. Receive B1 inhomogeneity was reduced using ANTs’ N4BiasFieldCorrection8. Magnitude images and simulated FA distributions over the VOI were plotted to assess image quality.Results
Figure 3 shows magnitude images in three orientations for volunteers 1-5 using CP, phase shimming and kt-points. CP shows clear signal dropouts, which are removed using phase shimming and kt-points. The kt-points scans show an increased overall contrast over phase shimming and CP. These results match with the simulations. They show that with both phase shimming and kt-points, the lowest FAs in CP can be increased, eliminating signal dropouts. Kt-points is able to deliver the nominal FA of 8˚ with a smaller FA range, while phase shimming only reaches a mean of ~4˚. The same behavior is seen in all five volunteers. Figure 4 shows good intra- and inter-session repeatability for volunteer 5. In volunteers 2 and 6, one of the scans showed signal dropouts, despite promising simulation results. Volunteer 7 reported breath-hold difficulties and showed varying B1 patterns.Discussion
With FPE-DREAM, a homogeneous small-flip-angle whole-liver GRE can be acquired at 7T using kt-points pulses. With phase shimming, signal dropouts could be removed but with roughly half the nominal flip angle over the VOI. Occasionally, kt-points acquisitions give residual signal dropouts as shown in Figure 4. From volunteers 2 and 6 it follows that repeated acquisitions led to successful shimming, pointing at motion or inconsistent breath-holds as a possible cause. This would also explain signal dropouts in volunteer 7. The BMI values of volunteers in this study were quite low. However, Figure 2a shows that artifact-free B1+ maps and good simulation results could also be achieved on a volunteer with a higher BMI, which is promising for successful application of kt-points. This will be subject of future research, as will be the application of universal pulses9 to avoid the time-consuming B1+ calibration phase. Finally, we intend to make the kt-points pulses slab-selective to enable smaller FOVs which, in combination with undersampling techniques like SENSE or CS10, could increase spatial resolution.Conclusion
With FPE-DREAM, we could acquire a homogeneous small-flip-angle whole-liver GRE at 7T using kt-points, with highly increased homogeneity and mean FA compared to phase shimming and CP.Acknowledgements
No acknowledgement found.References
- Cloos MA, Boulant N, Luong M, Ferrand G, Giacomini E, Le Bihan D, Amadon A. kT-points: short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson Med. 2012;67(1):72-80. doi: 10.1002/mrm.22978.
- Nehrke K, Versluis MJ, Webb A, Börnert P. Volumetric B1(+) mapping of the brain at 7T using DREAM. Magn Reson Med. 2014;71(1):246-56. doi: 10.1002/mrm.24667.
- Tse DHY, Poole MS, Magill AW, Felder J, Brenner D, Jon Shah N. Encoding methods for B1(+) mapping in parallel transmit systems at ultra high field. J Magn Reson. 2014;245:125-32. doi: 10.1016/j.jmr.2014.06.006.
- Raaijmakers AJE, Italiaander M, Voogt IJ, Luijten PR, Hoogduin JM, Klomp DWJ, van den Berg CAT. The Fractionated Dipole Antenna: A New Antenna for Body Imaging at 7 Tesla. Magn Reson Med. 2016;75:1366-1374. doi: 10.1002/mrm.25596
- Grissom WA, Khalighi M, Sacolick LI, Rutt BK, Vogel MW. Small-tip-angle spokes pulse design using interleaved greedy and local optimization methods. Magn Reson Med. 2012;68(5):1553-62. doi: 10.1002/mrm.24165.
- Roos T, Knapen T, van der Zwaag W. Reducing B1+ inhomogeneities in the brain at 7T: Pads or Parallel transmit? Proceedings of the 11th Annual Meeting ISMRM Benelux Chapter, 2019; Leiden, Netherlands
- Uecker M. Berkeley Advanced Reconstruction Toolbox Target Audience: 2015;163(1991):25176. doi:10.1002/mrm.25176.
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: Improved N3 Bias Correction. IEEE Trans Med Imag. 2010;29(6):1310-1320. doi: 10.1109/TMI.2010.2046908
- Gras V, Vignaud A, Amadon A, Le Bihan D, Boulant N. Universal pulses: A new concept for calibration-free parallel transmission. Magn Reson Med. 2016;77(2):635-643. doi: 10.1002/mrm.26148
- Lustig M, Donoho D, Pauly JM. Sparse MRI: The Application of Compressed Sensing for Rapid MR Imaging. Magn Reson Med. 2007;58:1182-1195. doi: 10.1002/mrm.21391.
Figures
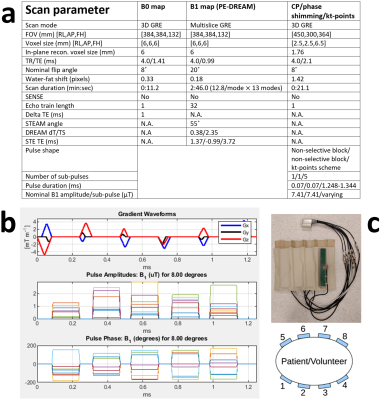
Figure 1: a) Scan parameters for B0 mapping, B1+ mapping and the scans using CP, phase shimming and kt-points. For scans utilizing kt-points, the echo time was defined as the time between the echo and the middle of the combination of sub-pulses. b) An exemplary (volunteer 2) kt-point pulse trajectory (gradient blip diagrams) and pulse amplitudes and phases. c) The eight-channel fractionated dipole antenna body array used for parallel transmission.
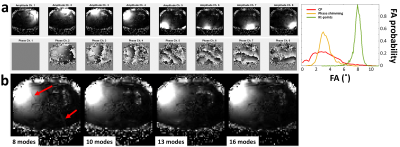
Figure 2: FPE-DREAM B1+ mapping in a volunteer with high BMI. a) Eight-channel B1+ amplitude and phase maps acquired using 13 modes (left) and corresponding whole-liver FA distribution simulations (right). The B1+ maps show no severe artifacts and the simulations show high homogeneity around the nominal FA for kt-points. b) Single-channel B1+ magnitude maps calculated from 8, 10, 13 and 16 modes. Maps calculated from 8 modes show artifacts (red arrows) that are removed when using >10 modes.
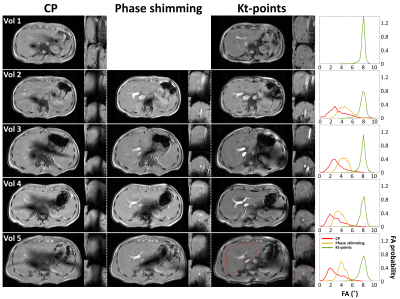
Figure 3: Magnitude images in three orientations for volunteers 1-5 using CP, phase shimming and kt-points (left). Slices are centered on the most pronounced signal dropout area in CP near the inferior vena cava. The clear signal dropouts in CP are removed using phase shimming and kt-points. Kt-points shows increased contrast over phase shimming and CP which was expected from the simulations (right). An exemplary whole-liver VOI used in pulse calculation is drawn in red (volunteer 5, kt-points).
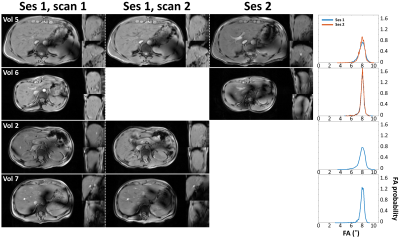
Figure 4: Robustness assessment. Magnitude images in three orientations (left) and simulations (right) of intra- and intersession kt-points repeatability tests in four volunteers. Volunteer 5 shows good repeatability in achieving whole-liver homogeneous FA. Volunteers 2, 6 and 7 show the occurrence of residual signal dropouts despite promising simulation results. Note that intra-session repetitions do not involve calculation of a new kt-points pulse and hence no separate simulation result.
DOI: https://doi.org/10.58530/2022/2868