2863
Mechanisms of relaxation in blood - Nuclear magnetic resonance dispersion profile of rat’s blood, plasma, and red cells at different temperatures1Université Paris Saclay, CEA, CNRS, Inserm, Laboratoire d’Imagerie biomédicale multimodale Paris Saclay (BioMaps), Orsay, France, 2Université Paris-Saclay, CEA, CNRS, Baobab, NeuroSpin, Gif-sur-Yvette, France, 3Aberdeen Biomedical Imaging Centre, University of Aberdeen, Foresterhill, AB25 2ZD, Aberdeen, UK, Aberdeen, Scotland, 4Laboratoire PHysico-chimie des Electrolytes et Nanosystèmes InterfaciauX, PHENIX, Sorbonne Université, CNRS, Paris, France
Synopsis
Measuring whole blood relaxation time is as a reliable diagnosis tool. Usually, one compartmental model is used to fit data, which seems a very simplistic approach considering the complexity of blood. The aim of this work is to test the validity of this assumption by studying the relaxation rate of whole blood, plasma and red blood cells (rbc) of rats at different temperatures. The whole blood is well described by a two-compartment approach, one corresponding to plasma and another for red blood cells. Once frozen, there is only one relaxation rate population, which is considered to be the rbc population.
Purpose
Field Cycling Imaging is an emerging technology designed to image molecular dynamics by exploring a range of magnetic field strengths during the scanning procedure1. This technique can help quantify changes in cellular water dynamics or tissue structure, providing complementary diagnostic information to current imaging methods2. We aim to better understand the FCI-NMR relaxation properties of human blood for early monitoring of tissues damages such as hemorrhages involved in severely premature infants. This requires a proper understanding of human blood NMR relaxation phenomena. Previous studies3 on whole blood samples assumed mono-exponential behavior of the magnetization curves acquired for Nuclear Magnetic Resonance Dispersion (NMRD) profiles. This implies a fast exchanges conditions between the two major blood compartments (red blood cells and plasma) that was verified only at low field. This work aims to validate the one or two compartments approach of the blood NMRD profiles.To that end, we analyzed whole rat blood, isolated plasma, and isolated red blood cells at different temperature, the idea being of decreasing the temperature until the extracellular compartment of whole blood was frozen, thus isolating the signal of the red blood cells.Method
Whole blood (WB), plasma, and red blood cells (RBC) samples (~2ml) were drawn from Sprague Dewley or F344 rats. Experiments were performed on a SPINMASTER2000 fast field cycling (FFC, Stelar®, Italy). Acquisitions on plasma and RBC samples were conducted at 4°C, and between 4°C and -9°C for the WB samples, this allowed the latter to transit from the liquid to the solid state. For each sample, we calculated the magnetization from the free induction decay signal for different values of magnetic fields $$$B_{relax}$$$ between 0.2 mT and 0.4 T and incrementation time $$$\tau$$$ between 0.04 ms and 2.4 s. The magnetization curves were then adjusted considering a model depending on the relaxation rates $$$R_1^{(k)}$$$ and the population $$$\alpha$$$ of one ($$$\alpha=1$$$) or two compartments:$$$M_z(\tau) = A\left[ \alpha_x e^{-R_1^{(1,x)}\tau} + (1-\alpha_x) e^{-R_1^{(2,x)}\tau} \right],$$$
where $$$A$$$ is a scaling parameter, $$$\alpha_x \in [0, 1]$$$ is the population of the first compartment, and $$$x$$$ denotes the sample (WB, plasma or RCB).
Results
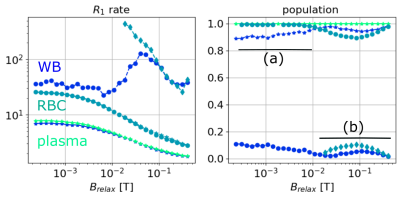
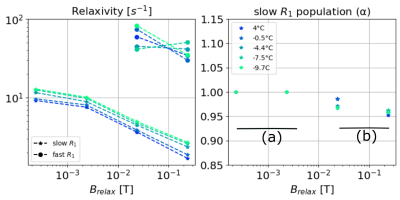
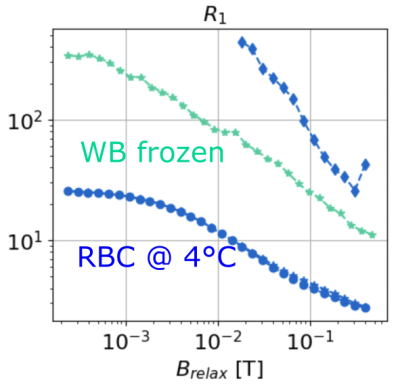
In Fig. 1, we present the NMRD profiles of WB, plasma, and RBC at 4°C. The plasma (green) has one unique compartment with a relatively slow $$$R_1^{(1,plasma)}$$$ value. The RBC sample (cyan) is described by (a) one unique population at low fields ($$$B_{relax}<20$$$ mT) and (b) two populations at higher field, with the new compartment (cyan diamond) being about 10% of the total population. Finally, the WB sample (blue) displays two compartments: a large population ($$$\alpha_{WB} > 0.9$$$) with a slow relaxation rate $$$R_1^{(1,WB)}$$$ close to that of and the plasma $$$R_1^{(1,plasma)}$$$, and a faster $$$R_1^{(2,WB)}$$$ close to $$$R_1^{(1,RCB)}$$$ at low field, and close to $$$R_1^{(2,RCB)}$$$ at higher field. In the Fig. 2 we present the NMRD profiles of the WB sample for temperature decreasing from 4°C to -9.7°C. As observed at 4°C, NRMD profiles displays two compartments: a large population ($$$\alpha_{WB} > 0.9$$$) with a slow relaxation rate $$$R_1^{(1, WB)}$$$, and a faster $$$R_1^{(2,WB)}$$$ at low field. Finally, in Fig. 3, we compare the NMRD profiles of the WB sample at -9°C (frozen state) with the RBC sample at 4°C. The two-compartment hypothesis, still valid in the liquid state, is no longer valid in the solid state. The $$$R_1^{(1,WB)}$$$ measured is of the same order than the ones of RBC at 4°C.Discussion and conclusion
It can be concluded that the two-compartment approach describes the whole blood well; one compartment with a slow relaxation rate corresponding to the plasma, and another faster to the red blood cells. There appears to be two compartments in the RCBs for fields above 10 mT. The higher relaxation rate of protons allows them to interact in a more complex way with the medium. Cooling the blood sample does not change this model as long as the blood remains in its liquid state. Once frozen, the visible population can be considered to be the RBC population.Acknowledgements
This work was financially supported by the ANR (ANR-20-CE19-0027) and the LabEx LaSIPS (ANR-10-LABX-0032-LaSIPS) managed by the French National Research Agency as part of the "Investissements d'avenir" programme (ANR-11-IDEX-0003)
References
1) Lurie, D.J., Aime, S., Baroni, S., Booth, N.A., Broche, L.M., Choi, C.H., Davies, G.R., Ismail, S. and Pine, K.J., 2010. Fast field-cycling magnetic resonance imaging. Comptes Rendus Physique, 11(2), pp.136-148.
2) Kimmich R, Anoardo E. Field-cycling NMR relaxometry. Prog. Nucl. Magn. Reson. Spectrosc. 2004;(44:257–320.3)
3) M. D. Herbst and J. H. Goldstein. American Journal of Physiology-Cell Physiology 1989 256:5, C1097-C1104