2858
SPION relaxivity at low field strengths with hyperpolarized 129Xe1University of North Carolina Chapel Hill, Chapel Hill, NC, United States
Synopsis
We provide superparamagnetic iron oxide longitudinal relaxivity measurements at both high (11.7 T) and low (0.6 – 2.1 mT) magnetic field strengths, for both 1H and hyperpolarized 129Xe nuclei. These measurements show significant increase in relaxivity at low field for both nuclei. We also provide some preliminary in vivo results for hyperpolarized 129Xe that suggest a depolarization mechanism similar to that of SPIONs from the hemoglobin found in whole blood.
Introduction
In clinical high-field MRI, superparamagnetic iron oxide contrast agents (SPIONs), which primarily affect T2/T2* relaxation processes of nearby spins, have been widely used for different molecular MRI applications.1-2 In particular, the combined use of SPIONs and hyperpolarized (HP) gases, has been shown to be of particular advantage for the detection of sub-millimeter cancer metastases in the lungs, where traditional 18F-FDG-PET lack both resolution and specificity.3 At high magnetic field strengths the effect of SPIONs is primarily on the transverse relaxation of 129Xe, with negligible effect on the longitudinal relaxation.4Recently, significant 1H R1 enhancement has been shown at low field.5 The scope of this work was to investigate whether similar R1 enhancement is also found for HP 129Xe at low magnetic field strengths. The results obtain for SPIONs may also help to explain the absence of a dissolved-phase xenon peak at low magnetic field strengths.
Methods
The relaxivity of SPIONs was measured in vitro at high and low magnetic field strengths for 1H and HP 129Xe nuclei. For the latter, relaxivity was investigated for both the gas- and dissolved-phase resonances, which can be easily differentiated at low field given the ~200 ppm difference in chemical shift between the two. High field studies were performed on a high resolution 500 MHz NMR spectrometer, while low field studies were performed on a home-built, variable-field NMR spectrometer operating at 25 kHz.6 At high field, inversion recovery sequences were used to measure 1H T1 values in different suspensions of SPIONs and deuterated water (99% D2O 1% H2O). At low field, a pre-polarization scheme was employed followed by a variable delay before excitation pulse transmission and signal acquisition. For 129Xe T1 measurements at high and low field, a HP compatible ventilator was used to deliver the hyperpolarized 129Xe gas (P ~ 15%) to either a suspension of SPION in DI water or an empty NMR tube with SPIONs attached to the inner surface to simulate the environment of the gas-phase in vivo.7 A small flip angle sequence was used to probe the magnetization as a function of time and the data was fit to account for the depletion of the magnetization caused by the RF excitations.8For the in vivo measurements, HP 129Xe gas was first dissolved into saline. Then, approximately 4 mL of the HP gas saturated saline was injected through the tail vein catheter of a rat placed with the heart and lungs positioned on the center of a surface coil in the 2.1 mT magnetic field. A series of excitation pulses were applied right after the injection, for approximately 10 seconds, to probe the 129Xe magnetization transferred from blood to the hearth and lungs. The same experiment was repeated three times.
Results
The in vitro results show a significant increase of the SPION relaxivity at low field strengths for both nuclei. A 200-fold increase in the 1H r1 was found at 0.6 mT compared to measurements made at 11.7 T, which agrees with previous studies on 1H SPION relaxivity at low field strengths.5 In comparison to measurements made at 11.7 T, a 23-fold and 10-fold increase in the r1 value for gas and dissolved-phase HP 129Xe was found at 2.1 mT, respectively. For the gas-phase signal, the reduction in T1 should be seen as an average as most spins are far from the SPIONs during measurement, which may explain the relatively weak effect given the concentrations used.For the in vivo low field study, neither a gas-phase nor a dissolved-phase signal was detected from the heart or lungs of the animal, despite the large volume of HP gas saturated saline injected intravenously. Similar measurements at high field have shown a predominant gas-phase signal from the lungs following IV injection of saline pre-saturated with HP Xe gas. 9 In our case, neither the gas-phase nor a dissolved-phase signal was detected. While the absence of a dissolved-phase signal can be easily explained by the relatively short T2 of 129Xe in blood, which at high field has been measured to be on the order of a few milliseconds, much shorter than the ring-time of our tuning circuit, the absence of a gas-phase signal from these in-vivo experiments suggests that at low field strengths the spins are depolarized in the blood before reaching the lungs.10
Conclusion
While at high magnetic field strengths SPIONs predominately affect the transverse relaxation time of nearby spins, at low magnetic fields the effect is predominantly on the longitudinal relaxation time of the gas, which could increase HP 129Xe sensitivity to SPIONs lodged in the lungs. As for 1H, the enhanced T1 relaxivity of 129Xe observed at low field strengths is due to a better frequency match between the magnetic fluctuations of SPIONs and the Larmor precession.The in vivo results presented here suggest a similar depolarization effect at low field from blood. The iron rich hemoglobin molecules may still experience large enough magnetic fluctuations resulting in a significant reduction in T1 compared to values at high field strengths. As a result, for dissolved-phase in vivo studies, it may be necessary to employ a carrier that prevents xenon depolarization in blood.
Acknowledgements
This work was partially supported by the NIH grant number R01DK108231, R01DK123206, and R21EB031319; Dr. Branca’s startup fund; and the NSF Graduate Research Fellowship DGE-1650116.References
1. Strobel, K. et al. Early detection of lung inflammation: Exploiting T1-effects of iron oxide particles using UTE MRI. Magn. Reson. Med. 68, 1924–1931 (2012).
2. Vignaud, A. et al. Magnetic susceptibility matching at the air-tissue interface in rat lung by using a superparamagnetic intravascular contrast agent: Influence on transverse relaxation time of hyperpolarized helium-3. Magn. Reson. Med. 54, 28–33 (2005).
3. Branca, R. T. et al. Molecular MRI for sensitive and specific detection of lung metastases. Proc. Natl. Acad. Sci. U. S. A. 107, 3693–3697 (2010).
4. Burant, A., Antonacci, M., McCallister, D., Zhang, L. & Branca, R. T. Effects of superparamagnetic iron oxide nanoparticles on the longitudinal and transverse relaxation of hyperpolarized xenon gas. J. Magn. Reson. 291, 53–62 (2018).
5. Yin, X. et al. Large T1 contrast enhancement using superparamagnetic nanoparticles in ultra-low field MRI. Sci. Rep. 8, 1–10 (2018).
6. Bryden, N., Antonacci, M., Kelley, M. & Branca, R. T. An open-source, low-cost NMR spectrometer operating in the mT field regime. J. Magn. Reson. 332, 107076 (2021).
7. Nouls, J. A Constant-Volume Ventilator and Gas Recapture System for Hyperpolarized Gas MRI of Mouse and Rat Lungs. Concepts Magn. Reson. Part B Magn. Reson. Eng. 49B, 78–88 (2011).
8. Puckeridge, M., Pagès, G. & Kuchel, P. W. Simultaneous estimation of T1 and the flip angle in hyperpolarized NMR experiments using acquisition at non-regular time intervals. J. Magn. Reson. 222, 68–73 (2012).
9. Driehuys, B., Moller, H. E., Cleveland, Z. I., Pollaro, J. & Hedlund, L. W. Pulmonary Perfusion and Xenon Gas Exchange in Rats: MR Imaging with Intravenous Injection of Hyperpolarized 129Xe. Radiology 252, 386–393 (2009).
10. Wilson, G. J., Santyr, G., Anderson, M. E. & PM DeLuca, J. T2 of 129Xe in Rat Tissue Homogenates and Blood at 9.4 T. Proc. Int. Soc. Magn. Reson. Med. 7, 2102 (1999).
Figures
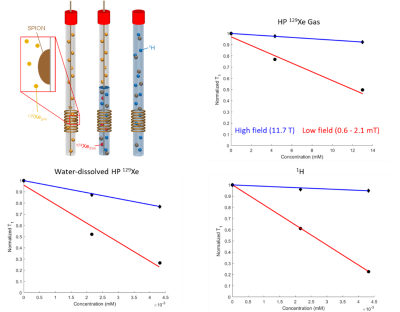
Figure 1: Experimental setup (top left). For 129Xe gas measurements, SPIONs were attached on the inner surface of an NMR tube to which gas was dispensed using an HP-compatible ventilator. For water-dissolved 129Xe measurements, gas was bubbled directly into a suspension of DI water containing SPION at different concentrations. Plots showing reduction in T1 as a function of SPION molar concentration for HP 129Xe gas (top right), water-dissolved HP 129Xe (bottom left), and 1H (bottom right).
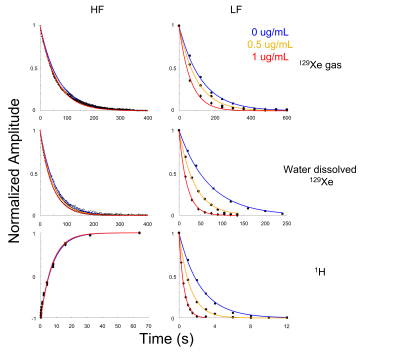
Figure 2: Data and fits for T1 relaxation measurements done at low (left) and high (right) magnetic field strengths. Relaxation for both nuclei was measured for 3 concentrations of SPIONs. For 1H, a pre-polarization scheme was employed at low field, while at high field an inversion recovery sequence was used. For both dissolved and gas-phase 129Xe, a small flip angle sequence was employed to probe the non-renewable polarization as a function of time. Note the more significant change in T1 relaxation as a function of SPION concentration at lower magnetic field strengths.
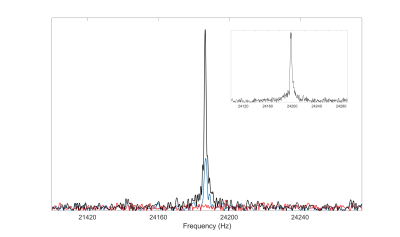
Figure 3: Main plot shows saline-dissolved HP 129Xe signal from the syringe before (black) and after (blue) injection. No dissolved-phase or gas-phase signal was observed from the rat post injection (red). Total signal loss suggests rapid depolarization of HP 129Xe in blood at this low field strength (2.1 mT). Inset plot shows the typical gas-phase signal acquired at the same field strength from a mouse ventilated with HP 129Xe gas, proving the instrument is capable of detecting much smaller volumes of HP 129Xe in the lungs as long as sufficient polarization is preserved.