2851
The CALIPR framework comprehensively improves acquisition, reconstruction & analysis of multi-component relaxation imaging1Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 2International Collaboration on Repair Discoveries, University of British Columbia, Vancouver, BC, Canada, 3Radiology, University of Pennsylvania, Philadelphia, PA, United States, 4MR Clinical Science, Philips Canada, Mississauga, ON, Canada, 5Radiology, University of British Columbia, Vancouver, BC, Canada, 6Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada, 7UBC MRI Research Centre, University of British Columbia, Vancouver, BC, Canada, 8Medicine (Neurology), University of British Columbia, Vancouver, BC, Canada
Synopsis
We introduce the CALIPR framework, applied here to multi-component T2 relaxation myelin water imaging (MWI) to achieve a simultaneous increase in data quality and decrease in acquisition time. CALIPR showed robust results for data acquired with aggressive under sampling acceleration, strong agreement with a newly developed “gold-standard” reference MWI acquisition, excellent scan-rescan repeatability, and the ability to acquire whole-brain MWI in under 5 minutes.
While this work demonstrates dramatic improvements the CALIPR framework brings to MWI, its utility can be extended to a wide range of both quantitative and qualitative MRI applications across different scanner vendors and field strengths.Introduction
Multi-component quantitative T2 mapping techniques can provide valuable in-vivo biomarkers, such as the highly specific myelin water fraction (MWF) metric produced by myelin water imaging (MWI)1,2. Here we introduce the Constrained, Adaptive, Low-dimensional, Intrinsically Precise Reconstruction (CALIPR) framework, and demonstrate its utility as a comprehensive approach for rapid and robust acquisition, reconstruction, and analysis of multi-component quantitative T2 relaxation data.Methods
Reference MWIAll data was acquired at 3T with a 32-channel head coil (Philips Elition X).
Development: A “gold-standard” reference MWI sequence was developed using a post-mortem, single-hemisphere brain sample from a subject with multiple sclerosis, which facilitated sequence optimization without motion artefacts and validation in the presence of unique relaxation times associated with tissue fixation and pathological processes. In-vivo data was also acquired from a healthy volunteer (male, 25yrs).
Acquisition: The optimized reference acquisition used a 3D multi-echo spin-echo sequence; Fixed brain: 56 echoes, echo spacing=5.6ms, TR=1252ms, acquired resolution=1.7x1.7x1.7mm3, reconstructed resolution=1.5x1.5x1.5mm3, field-of-view=240x192x100mm3, fully sampled acquisition time=2h:47m:25s, conservative compressed sensing (CS) under sampling with acceleration factor=1.3, 2h:8m:10s (Figure 1A).
Parameters were similar for the healthy volunteer reference scan except: echo spacing=5.2ms, TR=1277ms, acceleration factor=1.8, 1h:34m:22s (Figure 1B).
Both datasets were reconstructed with the default product reconstruction and post-processing.
CALIPR Acquisition
A modified version of the University of Amsterdam Academic Medical Center (AMC) ‘PROspective Undersampling in multiple Dimensions’ (PROUD) software patch3,4 was used to leverage redundancy in the data by prospectively under sampling with temporally-incoherent sampling schemes (variable-density Poisson distribution projected onto a uniform under sampling grid with an elliptical k-space shutter). Fully sampled central calibration regions at early echo times ensured sufficient characterization of stimulated echoes effects, required for accurate estimation of refocusing flip angles and T2 relaxation components during analysis.
CALIPR Reconstruction
Figure 2 outlines the CALIPR reconstruction process: 1) a conventional CS reconstruction generates echo time images 2) used to generate a subspace based on principal components of the signal evolution across echo times, before 3) a subspace-constrained (SC) reconstruction generates coefficient images corresponding to amplitudes of each subspace component 4) which are projected from their low-dimensional representation into echo time images, used for quantitative analysis. Unlike conventional SC reconstruction approaches, the CALIPR subspace is adaptive, based on each dataset itself rather than simulated signal evolutions generated a priori using a signal model. To reduce processing times, reconstructions were solved with BART5 using FISTA6 on GPU for k-space data compressed to 8 virtual channels7, with coil sensitivity maps estimated using ESPIRiT8.
Multi-Component T2 Image Analysis
Kumar et al. previously introduced a multi-component T2 analysis algorithm9, which uses spatial as well as temporal regularization to provide improved accuracy, robustness to noise, and inter-sequence correlation for the resulting quantitative metrics, albeit at the cost of prohibitively long processing time. Here we present an improved, much faster version, with relaxed criteria for refinement of the spatial regularization factors and fewer iterations used to refine the maps.
Results and Discussion
Reference MWI Acquisition with Improved AnalysisFigure 1 shows that MWF maps generated with the improved analysis demonstrate markedly better noise robustness, compared to temporal regularization only, even for high signal-to-noise ratio reference data with acquisition time >2 hours. Our modifications drastically reduced the total processing time from days to ~3 hours (24 CPUs) but had a negligible effect on the results compared to the original (MWF mean absolute error=0.36% in brain).
Retrospective Under Sampling: Validation
Figure 3 compares the fixed brain reference with CS, SC, and CALIPR reconstructions of retrospectively under sampled reference data (acceleration factor=14.6). While SC and CALIPR reconstructions both produced high-quality images, CALIPR had improved detail and reduced propagation of noise-like under sampling artifacts into the MWF map, even after attempting to optimize the simulated signal evolutions used to generate the SC reconstruction subspace.
Prospective Under Sampling: Scan-Rescan Repeatability for CALIPR MWI
Figure 4 compares the healthy volunteer reference with two CALIPR acquisitions (acceleration factor=22.1, 7m:34s) acquired during the same scan session more than an hour apart. The CALIPR images show sharp tissue contrast and detail and the MWF maps demonstrate excellent qualitative repeatability.
Prospective Under Sampling: Sub 5-Minute CALIPR MWI
Figure 5 demonstrates the ability to acquire robust, full-brain MWI data in less than 5 minutes using the CALIPR MWI sequence acquired at 2.0x2.0x2.0mm3 resolution (acceleration factor=24.8, 4m:53s).
Conclusion
Compared to commonly used multi-component quantitative T2 relaxation imaging10, the CALIPR sequence provides improved quantification of short-T2 components at higher resolution with reduced orientation dependence11, specific absorption rate, peripheral nerve stimulation, and acoustic noise. Self-contained use of the acquired data to generate the subspace and coil sensitivity maps avoids the need to generate an appropriate signal model or acquire and export external pre-scans when implementing CALIPR for different applications or MRI vendors, respectively.By simultaneously improving data quality for research applications and reducing acquisition time for clinical applications, the CALIPR framework facilitates multi-component quantitative T2 mapping without compromise and negates the need to settle for acquiring less-informative MRI. While this work demonstrates the dramatic improvements that the CALIPR framework brings to MWI, its utility can be extended to a plethora of both quantitative and qualitative MRI applications across different scanner vendors and field strengths.
Acknowledgements
We sincerely thank the MS patient and family for tissue donation. For this study, we used a modified version of the AMC ‘PROspective Undersampling in multiple Dimensions’ (PROUD) software patch. Adam Dvorak is supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Alexander Graham Bell Canada Graduate Scholarship. Shannon Kolind is supported by a Michael Smith Foundation for Health Research Scholar award and the Milan and Maureen Ilich Foundation. Additional funding support was provided by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant [F17-05113].
References
1 MacKay, A. et al. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med 31, 673-677 (1994).
2 Laule, C. et al. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler 12, 747-753, doi:10.1177/1352458506070928 (2006).
3 Peper, E. S. et al. Highly accelerated 4D flow cardiovascular magnetic resonance using a pseudo-spiral Cartesian acquisition and compressed sensing reconstruction for carotid flow and wall shear stress. J Cardiovasc Magn R 22, doi:ARTN 7 10.1186/s12968-019-0582-z (2020).
4 Gottwald, L. M. et al. Pseudo-spiral sampling and compressed sensing reconstruction provides flexibility of temporal resolution in accelerated aortic 4D flow MRI: A comparison with k-t principal component analysis. NMR Biomed. 33, doi:ARTN e4255
10.1002/nbm.4255 (2020).
5 Uecker, M. The Berkeley Advanced Reconstruction Toolbox (BART). DOI: 10.5281/zenodo.592960. doi:10.5281/zenodo.592960 (2019).
6 Beck, A. & Teboulle, M. A Fast Iterative Shrinkage-Thresholding Algorithm for Linear Inverse Problems. Siam Journal on Imaging Sciences 2, 183-202, doi:10.1137/080716542 (2009).
7 Zhang, T., Pauly, J. M., Vasanawala, S. S. & Lustig, M. Coil compression for accelerated imaging with Cartesian sampling. Magnetic Resonance in Medicine 69, 571-582, doi:10.1002/mrm.24267 (2013).
8 Uecker, M. et al. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med 71, 990-1001, doi:10.1002/mrm.24751 (2014).
9 Kumar, D. et al. Using 3D spatial correlations to improve the noise robustness of multi component analysis of 3D multi echo quantitative T2 relaxometry data. Neuroimage 178, 583-601, doi:10.1016/j.neuroimage.2018.05.026 (2018).
10 Prasloski, T. et al. Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. Neuroimage 63, 533-539, doi:10.1016/j.neuroimage.2012.06.064 (2012).
11 Birkl, C., Doucette, J., Fan, M., Hernandez-Torres, E. & Rauscher, A. Myelin water imaging depends on white matter fiber orientation in the human brain. Magnetic Resonance in Medicine 85, 2221-2231, doi:10.1002/mrm.28543 (2021).
Figures
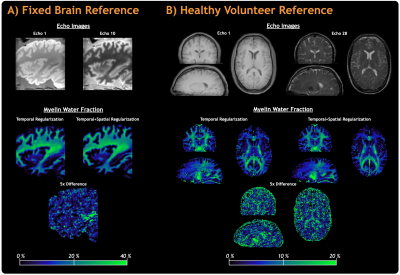
Figure 1: Reference myelin water imaging data acquired for fixed brain in 2h:8m:10s (Figure 1A) and for a healthy volunteer (male, 25 years) in 1h:34m:22s (Figure 1B) using conservative acceleration factors of 1.3 and 1.8, respectively. Both reference scans used product compressed sensing acceleration and default reconstruction/post-processing parameters. Quantitative myelin water fraction maps were calculated with the standard (temporal regularization) and improved (temporal+spatial regularization) analyses.
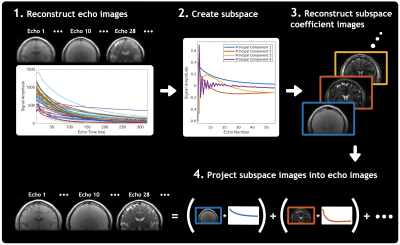
Figure 2: An overview of the CALIPR framework image reconstruction process.
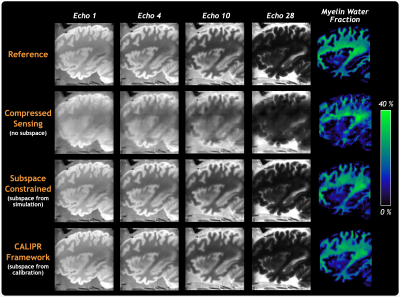
Figure 3: Comparison of images and MWF maps between the full fixed brain reference myelin water imaging dataset and retrospectively under sampled (factor 14.6) k-space data reconstructed using compressed sensing, subspace constrained, and CALIPR image reconstructions.
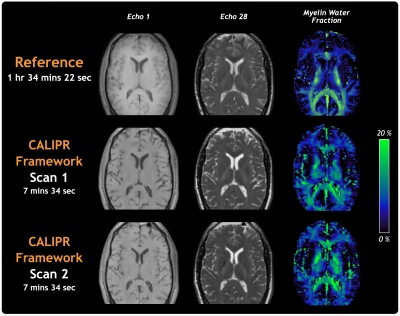
Figure 4: Comparison of images and MWF maps between the healthy volunteer reference myelin water imaging dataset and two prospectively under sampled CALIPR acquisitions (1.7 mm isotropic with acceleration factor 22.1 in 7m:34s), performed during the same scan session more than an hour apart.
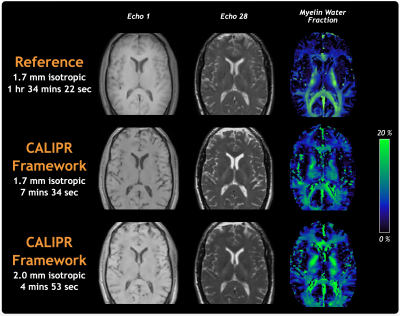
Figure 5: Comparison of images and MWF maps between the healthy volunteer reference myelin water imaging dataset and prospectively under sampled CALIPR acquisitions (1.7 mm isotropic with acceleration factor 22.1 in 7m:34s, 2.0 mm isotropic with acceleration factor 24.8 in 4m:53s) performed during different scan sessions.