2843
Distortion correction of 3D EPI for cortical depth-resolved fMRI at 7T1Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 3Centre de Résonance Magnétique des Systèmes Biologiques, CNRS‐University Bordeaux, Bordeaux, France
Synopsis
Geometric distortion is a major concern for cortical depth-dependent fMRI using EPI readouts that suffer from low bandwidth in the phase-encoded direction. Distortions are exacerbated at higher field strengths due to increased B0 field inhomogeneity. In this study, we quantitatively compared two distortion correction methods (B0 field-mapping and reversed-PE) applied to submillimeter (0.8mm isotropic) 3DEPI data at 7T. Cortical alignment was evaluated through comparison with an anatomically-faithful MP2RAGE reference by computing the dice coefficient and normalised mutual information. Both distortion correction methods usefully improve alignment. The reversed-PE approach performed better.
Introduction
Echo-planar imaging (EPI) is the most common fMRI readout but is prone to geometric distortions along the low bandwidth phase-encoded (PE) direction [1]. This is exacerbated at 7T by increased B0 field inhomogeneity and by the push to longer readouts necessary for higher resolution in cortical-depth-dependent analyses. Multiple methods have been suggested to reduce distortions, most notably based on estimating and correcting voxel displacements via B0 field-mapping or reversed phase-encoding (reversed-PE) [2-5]. However, comparisons of these techniques in the context of fMRI have previously only been performed either at lower field or lower resolution.Here we mitigated geometric distortions at the point of acquisition by using in-plane segmentation (factor 2) and parallel imaging (factor 4). 3DEPI was utilised instead of 2DEPI to gain SNR [6] and avoid slice profile effects. Magnetisation transfer (MT) weighting was added to boost contrast between tissue types. Data were post-processed with both B0 field-mapping and reversed-PE correction techniques, and the resulting cortical alignment evaluated against an anatomically-faithful MP2RAGE reference.
Methods
Data acquisition: 21 participants were scanned at 7T (Siemens MAGNETOM Terra) using an 8 transmit and 32 receive channel head coil (Nova Medical). Following fMRI data acquisition (a memory recall paradigm), whole brain MT-weighted 3DEPI (MT-3DEPI) volumes were acquired with reversed PE polarity (PA then AP) and an EPI readout that matched the fMRI time-series. B0 field mapping and T1-weighted anatomical scans were also acquired using dual-echo gradient-echo and MP2RAGE sequences respectively (see Table 1).Data processing: All quantitative analyses were performed in the anatomical space of the individual, defined by their MP2RAGE. The common processing steps (Fig.1) included co-registration of the MT-3DEPI to the MP2RAGE using boundary-based registration [7], segmentation in SPM12 [8], and parcellation into 48 grey matter (GM) regions of interest (ROIs) based on the Harvard-Oxford cortical atlas [9]. B0 field-mapping incorporated brain masking to exclude edge voxels in the phase images and generation of the B0 field-map for unwarping using FEAT as implemented in FSL [10] (Fig.1b). For the reversed-PE correction, FSL’s topup and applytopup were used (Fig.1c).
Analysis: The effect of distortion correction was qualitatively assessed by boundary inspection and quantitatively by computing the dice coefficient (DC), indicating the degree of cortical overlap, and the normalised mutual information (NMI), indicating the degree of shared information, between the MT-3DEPI and MP2RAGE.
Results
Fig.2 shows the result of distortion correction for a representative individual. Both B0 field-mapping and reversed-PE techniques showed improvement in GM boundary alignment (orange arrows in prefrontal cortex). Reversed-PE outperformed B0 field-mapping (green arrows in zoomed view). However, some distortions remained regardless of technique (blue arrows).Fig.3a shows the DC for the 3 pipelines as a function of GM probability. Prior to correction, the DC was 0.771±0.021 (mean±standard deviation over thresholds and participants). This increased to 0.786±0.021 and 0.805±0.019 following B0 field-mapping and reversed-PE corrections respectively. This indicates more GM overlap between the MT-3DEPI and MP2RAGE data after correction, and more so for the reversed-PE method.
Fig.3b shows the relative improvement in NMI (corrected compared to non-corrected) for the B0 field-mapping and reversed-PE techniques. The reversed-PE method led to widespread and stronger improvements, particularly in prefrontal regions. In some regions, such as the central opercular, middle frontal and cingulate gyri, B0 field-mapping marginally (<1%) reduced NMI.
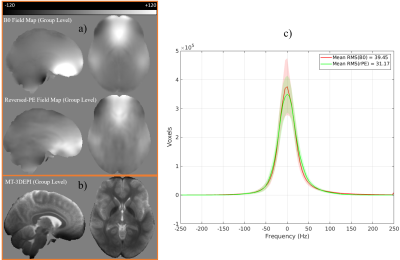
The average field maps (across participants, in Hz) estimated by each correction method are qualitatively similar (Fig.4a). A notable discrepancy was visible in the inferior frontal cortex, which suffered from signal dropout in the MT-3DEPI data (Fig.4b) leading to substantial under-estimation of the off-resonance relative to the B0 field-mapping approach. The mean (across participants) root-mean-squared frequency distribution values were 39.45Hz and 31.17Hz for the B0 field-mapping and reversed-PE methods, respectively (Fig.4c).
Discussion
In this study, we quantitatively compared B0 field-mapping and reversed-PE based distortion correction in the context of sub-millimeter 3DEPI. DC and NMI revealed that both methods improve alignment between MT-3DEPI and an anatomically-faithful MP2RAGE reference. Furthermore, reversed-PE outperformed B0 field-mapping, consistent with a recent study examining lower resolution 2DEPI at 7T [4].B0 field-mapping performance may have been hindered near brain edges where phase changes are rapid and poor signal-to-noise ratio (SNR) affects phase-unwrapping. Furthermore, the field-mapping data were acquired at lower resolution (2mm) and with limited contrast (sacrificed for SNR) whereas the MT-3DEPI data had matched 0.8mm resolution and enhanced contrast via an MT-prepulse. Nonetheless, B0 field-mapping still reduced distortions in most cortical regions. However, our analyses indicate that the reversed-PE approach is to be favoured.
MT-3DEPI is useful for post-processing of fMRI data, most notably for co-registration and segmentation. The additional acquisition time for the reversed-PE volume was approximately 1 minute 53s, which is less than that required for the B0 field-mapping sequence. In addition, the finding of improved distortion correction using reversed-PE generalises to the case of typical fMRI data by integrating a reversed-PE volume (~4s acquisition time) into the acquisition of restricted field-of-view 3DEPI time series data used for fMRI (data not shown).
Acknowledgements
The Wellcome Centre for Human Neuroimaging is supported by core funding from the Wellcome [203147/Z/16/Z]. E.A.M. is supported by a Wellcome Principal Research Fellowship [210567/Z/18/Z].References
1. Jezzard, P. and S. Clare, Sources of distortion in functional MRI data. Human brain mapping, 1999. 8(2‐3): p. 80-85.
2. Fritz, L., et al. Comparison of EPI distortion correction methods at 3T and 7T. in Poster Presented at the Annual Meeting of the Organization for Human Brain Mapping. 2014.
3. Yamamoto, T., et al., Quantitative Evaluations of Geometrical Distortion Corrections in Cortical Surface-Based Analysis of High-Resolution Functional MRI Data at 7T. Journal of Magnetic Resonance Imaging, 2021. 53(4): p. 1220-1234.
4. Schallmo, M.P., et al., Assessing methods for geometric distortion compensation in 7 T gradient echo functional MRI data. Human Brain Mapping, 2021. 42(13): p. 4205-4223.
5. Holland, D., J.M. Kuperman, and A.M. Dale, Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage, 2010. 50(1): p. 175-183.
6. Huber, L., et al., Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications. Neuroimage, 2018. 164: p. 131-143.
7. Greve, D.N. and B. Fischl, Accurate and robust brain image alignment using boundary-based registration. Neuroimage, 2009. 48(1): p. 63-72.
8. Acton, P.D. and K.J. Friston, Statistical parametric mapping in functional neuroimaging: beyond PET and fMRI activation studies. European Journal of Nuclear Medicine, 1998. 25(7): p. 663-667.
9. Desikan, R.S., et al., An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 2006. 31(3): p. 968-980.
10. Jenkinson, M., et al., Fsl. Neuroimage, 2012. 62(2): p. 782-790.
Figures
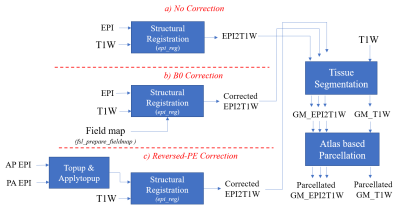
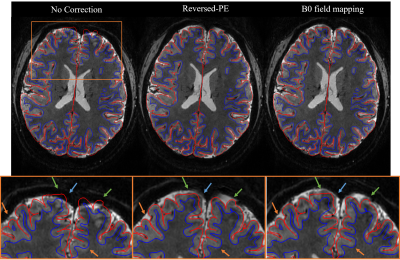
Figure 2. Distortion correction results for non-corrected, reversed-PE and B0 field-mapping pipelines for a representative participant. The GM (red) and WM (blue) edges of the MP2RAGE data are overlaid on the registered MT-3DEPI data to assess the correction visually. Below is a zoomed view (orange panel, top) of the MT-3DEPI data. The improvements afforded by both correction techniques (orange arrows), or specifically the reversed-PE method (green arrows), are highlighted. Some regions did not improve (blue arrow).
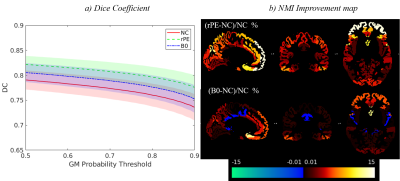
Figure 3. a) Dice coefficient between GM binary masks derived from the MP2RAGE and MT-3DEPI data as a function of GM probability threshold. The bold lines indicate the mean DC, across participants, while the shaded areas represent one standard deviation above and below this value. b) NMI improvement maps for both reversed-PE (top) and B0 field-mapping (bottom) correction techniques in 48 ROIs defined by the Harvard-Oxford cortical atlas. (NC: No-correction, rPE: reversed-PE, B0: B0 field-mapping).