2840
Removal of perfusion lag structure before ICA-FIX cleaning boosts both reproducibility and between-subject similarity in fcMRI1Laboratory for Brain Connectomics Imaging, RIKEN Center for Biosystems Dynamics Research, Kobe, Japan
Synopsis
Removal of the perfusion lag structure in the fMRI signal was combined with independent component analysis (ICA)-based cleaning to evaluate its effect on test-retest reproducibility. In HCP test-retest data from 40 subjects, either treatment and the combined method effectively improved the reproducibility of functional connectivity. However, the effect was more evident in between-subject similarity of the connectivity pattern than within-subject similarity. This finding suggests that the perfusion lag structure may be one of the sources of between-subject variability, that may degrade the functional connectivity.
Introduction
Given the replication crisis afflicting numerous scientific fields, reliable measurement is crucial. Reliability consists of both stability (or reproducibility) and accuracy (as a biomarker) that have been technical challenges in functional connectivity (FC). Resting-state fMRI analysis is based on correlation of random fluctuations without averaging, making it frail to non-neuronal components. To date, the lack of a gold standard for validation has hampered the establishment of a unified procedure despite the emerging solid techniques. A sophistication in multi-feature based ICA denoising (e.g., ICA-FIX) has achieved dramatic improvement in reproducibility by taking out various types of noise 1,2. Further removal of physiological noises that broadly affect the FC is a recent focus of attention 3–5.We previously reported that removal of the perfusion lag structure may improve task fMRI 6, as well as resting-state FC 7,8. This treatment deals with a moving phase of systemic low-frequency oscillation (sLFO) possibly originating from the cardiopulmonary autonomic loop 8. In the present study, we tested this technique, called “deperfusioning”, on the test-retest dataset from Human Connectome Project to evaluate its effect on reproducibility and between-subject similarity of FC.
Methods
In the HCP-YA test-retest paired data (N = 40), four denoising conditions were compared: data without denoising, with deperfusioning, with ICA-FIX, and with deperfusioning+ICA-FIX. All FIX denoising was performed after concatenating the 4 runs (multi-run FIX), using the uniform feature training file.Concatenation was done also for the perfusion lag mapping (Fig. 1). The deperfusioning procedure involved grouping of the brain voxels by this lag map, namely the phase of sLFO, at 0.72-s step. Each voxel group went through a regression by the sLFO with the corresponding phase, leaving the rest of slow components plus the full high frequency components (> 0.07 Hz). After the deperfusioning, ICA yielded smaller numbers of components due to reduced variance than those used non-deperfusioned data (Mean ± SD: 362.5 ± 113.7 vs 128.6 ± 88.7).
After the 4 types of preprocessing followed by mapping onto the surface, cortical parcellation was performed 9 to calculate every combination of FC across the 360 regions and create the connectivity matrix. Absolute agreement with sessions (days) as rows and voxels as columns (ICCbetween 10) was calculated for each correlation map of the 360 seeds and averaged as a measure of similarity, or reproducibility (Fig. 2).
Results and Discussion
Within-subject reproducibility between day-1 and day-2 was significantly improved by either denoising method of deperfusioning or ICA-FIX (Fig. 3 & 4), but the additional change by the combined method of deperfusioning+ICA-FIX cleaning was small. Interestingly, however, the combined method increased between-subject similarity of the FC networks. This finding was corroborated by the nature of the perfusion lag structure showing relatively low between-subject similarity (Fig. 5), or large interindividual variability, possibly due to variation of vascular anatomy. The present results therefore suggest the perfusion lag structure as a major source of inter-individual difference of non-neuronal origin.Further study is warranted to validate this combined procedure as a denoising pipeline. Accumulating evidence suggests an emergence of spatial correlation driven by non-neuronal, vascular physiological sources 11–13. The sLFO is intrinsic to blood content and can emerge without vessel diameter change, which was supported by the absent lag structure in fMRI using a contrast agent, USPIO 8,14,15. Removal of the lag structure may therefore be one of the feasible treatments to increase accuracy of fMRI 16,17; however, minor but possible desensitization to the neural components must be addressed in future studies. For example, reduced between-subject variability in the present result might involve removal of neuronal signals accidentally correlated with the sLFO. Comparison with another promising method, temporal ICA targeted for global noise removal, is also necessary 18. Ultimately, the validation should require non-BOLD techniques such as or CBV-based fMRI 19 with or without tasks or histology of axonal connection 20.
Acknowledgements
No acknowledgement found.References
1 Salimi-Khorshidi, Gholamreza, Douaud, Gwenaëlle, Beckmann, Christian F., Glasser, Matthew F., et al. (04/2014) ‘Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers’. NeuroImage, 90, pp. 449–468. [online] Available from: https://linkinghub.elsevier.com/retrieve/pii/S1053811913011956 (Accessed 29 November 2018)
2 Griffanti, Ludovica, Salimi-Khorshidi, Gholamreza, Beckmann, Christian F., Auerbach, Edward J., et al. (07/2014) ‘ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging’. NeuroImage, 95, pp. 232–247. [online] Available from: https://linkinghub.elsevier.com/retrieve/pii/S1053811914001815 (Accessed 19 August 2020)
3 Glasser, Matthew F., Coalson, Timothy S., Bijsterbosch, Janine D., Harrison, Samuel J., et al. (n.d.) ‘Using Temporal ICA to Selectively Remove Global Noise While Preserving Global Signal in Functional MRI Data: Main Supplementary Information’.
4 Chen, Jingyuan E., Lewis, Laura D., Chang, Catie, Tian, Qiyuan, et al. (2020) ‘Resting-state “physiological networks”’. NeuroImage, 213, p. 116707. [online] Available from: http://www.sciencedirect.com/science/article/pii/S1053811920301944 (Accessed 23 September 2020)
5 Tong, Yunjie, Hocke, Lia M., Fan, Xiaoying, Janes, Amy C. and Frederick, Blaise Deb (2015) ‘Can apparent resting state connectivity arise from systemic fluctuations?’ Frontiers in human neuroscience, 9. [online] Available from: http://www.frontiersin.org/Human_Neuroscience/10.3389/fnhum.2015.00285/abstract (Accessed 29 November 2018)
6 Aso, Toshihiko and Hayashi, Takuya (2020) ‘Effect of the perfusion lag structure removal on task fMRI’, in ISMRM2020. [online] Available from: https://cds.ismrm.org/protected/20MPresentations/abstracts/3815.html
7 Erdoğan, Sinem B., Tong, Yunjie, Hocke, Lia M., Lindsey, Kimberly P. and deB Frederick, Blaise (2016) ‘Correcting for Blood Arrival Time in Global Mean Regression Enhances Functional Connectivity Analysis of Resting State fMRI-BOLD Signals’. Frontiers in human neuroscience, 10. [online] Available from: http://journal.frontiersin.org/Article/10.3389/fnhum.2016.00311/abstract (Accessed 25 November 2018)
8 Aso, Toshihiko, Urayama, Shinnichi, Fukuyama, Hidenao and Murai, Toshiya (2019) ‘Axial variation of deoxyhemoglobin density as a source of the low-frequency time lag structure in blood oxygenation level-dependent signals’. PloS one, 14(9), p. e0222787. [online] Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0222787 (Accessed 24 September 2019)
9 Glasser, Matthew F., Coalson, Timothy S., Robinson, Emma C., Hacker, Carl D., et al. (2016) ‘A multi-modal parcellation of human cerebral cortex’. Nature, 536(7615), pp. 171–178. [online] Available from: https://www.nature.com/articles/nature18933 (Accessed 12 December 2018)
10 Raemaekers, M., Vink, M., Zandbelt, B., van Wezel, R. J. A., et al. (07/2007) ‘Test–retest reliability of fMRI activation during prosaccades and antisaccades’. NeuroImage, 36(3), pp. 532–542. [online] Available from: http://linkinghub.elsevier.com/retrieve/pii/S1053811907002157 (Accessed 29 November 2018)
11 Bright, Molly G., Whittaker, Joseph R., Driver, Ian D. and Murphy, Kevin (2018) Vascular physiology drives functional brain networks, Neuroscience. [online] Available from: http://biorxiv.org/lookup/doi/10.1101/475491 (Accessed 22 July 2019)
12 Das, Aniruddha, Murphy, Kevin and Drew, Patrick J. (2021) ‘Rude mechanicals in brain haemodynamics: non-neural actors that influence blood flow’. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 376(1815), p. 20190635. [online] Available from: https://royalsocietypublishing.org/doi/10.1098/rstb.2019.0635 (Accessed 25 January 2021)
13 Aso, Toshihiko and Hayashi, Takuya (2021) ‘Seed-based Correlation Analysis on Isolated Perfusion Lag Structure in the Resting-state fMRI Signal’, in HBM2021.
14 Tong, Yunjie, Hocke, Lia M. and Frederick, Blaise B. (2019) ‘Low Frequency Systemic Hemodynamic “Noise” in Resting State BOLD fMRI: Characteristics, Causes, Implications, Mitigation Strategies, and Applications’. Frontiers in neuroscience, 13, p. 787. [online] Available from: https://www.frontiersin.org/article/10.3389/fnins.2019.00787/full (Accessed 27 October 2019)
15 Aso, Toshihiko and Hayashi, Takuya (2020) ‘BOLD signal-based perfusion lag mapping in monkey brain’, in Annual Meeting of The Organization for Human Brain Mapping.
16 Aso, Toshihiko (n.d.) ‘Extraction and removal of the time-lag structure within 4D blood oxygenation level dependent (BOLD) signal MRI data’. BOLDLagMapping. [online] Available from: https://github.com/RIKEN-BCIL/BOLDLagMapping
17 deB Frederick, Blaise (n.d.) ‘The rapidtide package’. [online] Available from: https://github.com/bbfrederick/rapidtide
18 Glasser, Matthew F., Coalson, Timothy S., Bijsterbosch, Janine D., Harrison, Samuel J., et al. (11/2018) ‘Using temporal ICA to selectively remove global noise while preserving global signal in functional MRI data’. NeuroImage, 181, pp. 692–717. [online] Available from: https://linkinghub.elsevier.com/retrieve/pii/S1053811918303963 (Accessed 29 November 2018)
19 Lu, Hanzhang, Golay, Xavier, Pekar, James J. and van Zijl, Peter C. M. (08/2003) ‘Functional magnetic resonance imaging based on changes in vascular space occupancy’. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 50(2), pp. 263–274. [online] Available from: http://doi.wiley.com/10.1002/mrm.10519 (Accessed 29 November 2018)
20 Van Essen, David C., Donahue, Chad J., Coalson, Timothy S., Kennedy, Henry, et al. (2019) ‘Cerebral cortical folding, parcellation, and connectivity in humans, nonhuman primates, and mice’. Proceedings of the National Academy of Sciences of the United States of America, 116(52), pp. 26173–26180. [online] Available from: https://www.pnas.org/content/116/52/26173 (Accessed 6 July 2020)
Figures
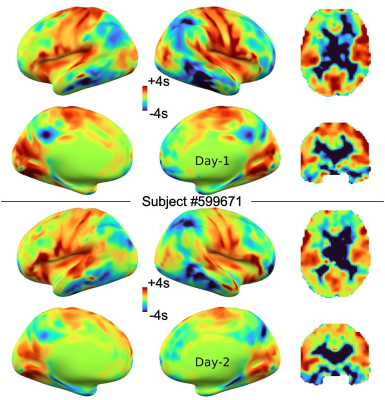
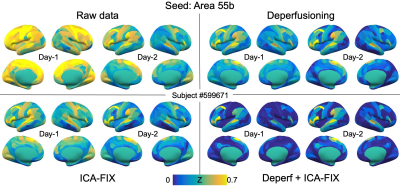
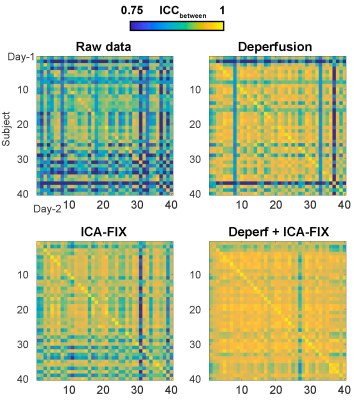
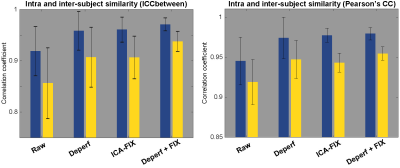
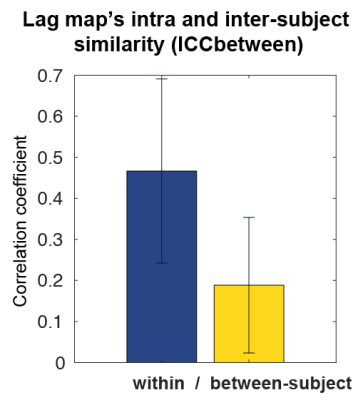
Figure 4. Left, Reproducibility (blue) improved by both deperfusioning and ICA-FIX, but the improvement by the combination of these two was small, although still significant (P = 0.005, paired-t test). The change was more prominent in the between-subject similarity (yellow).
Right, The same analysis was conducted for confirmation using Pearson’s correlation, which does not reflect absolute values, for the similarity measure. The coefficients were relatively higher than in ICC, but the results were essentially similar. Error bars show SD.