2831
Overcoming image distortions for DWI of prostate cancer using Spatiotemporal Encoding (SPEN)1Weizmann Institute of Science, Rehovot, Israel, 2Sheba Medical Center, Ramat Gan, Israel, 3Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 4Azrieli National Center for Brain Imaging, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Diffusion weighted imaging (DWI) plays an important role in prostate cancer early detection and characterization. These analyses commonly rely on single shot EPI acquisitions, which can be associated with image distortions induced by field inhomogeneities induced by tissue / air interfaces. Herein we compare the faithfulness of EPI-derived DWI data, against that arising from Spatiotemporal Encoding (SPEN). In general, we find that SPEN delivers more faithful DW representations of the prostate anatomy within comparable scan times, without compromising SNR. SPEN can thus improve the contrast for prostate lesion detection, both in DW images and in calculated ADC maps.
Introduction
Diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping are important components in the multiparametric clinical toolkit currently used for the detection and characterization of prostate lesions by MRI: The Prostate Imaging – Reporting and Data System Version 2.1 (PI-RADS v2.1).1 According to these recommendations, in addition to acquiring moderately b-weighted images (800-1000 s/mm2) plus another b-value between 0 and 1000 s/mm2 for more accurate calculation of ADC maps, the acquisition of images with b-weighting ≥1400 s/mm2 is recommended. This extensive b-value scheme coupled to the need for sufficient SNR –particularly for the latter images– as well as by constraints in the examination time allotted to acquire the DWI set, has led to the adoption of single shot spin-echo EPI for these exams. EPI’s immunity to distortions along the phase encoding (PE) direction, however, is limited by the minimum available echo spacing, prompting studies that have tried to tackle this problem by relying on either PROPELLER2 or on read-out segmented EPI acquisitions.3–5 Alternatively, SPatiotemporal ENcoding (SPEN) could be used as a method for these DWI acquisitions, as it shows increased immunity to magnetic field inhomogeneities and similar scan times to EPI acquisitions.6,7 The potential of single shot SPEN DWI and ADC mapping of prostate tumors in a clinical context, is herein assessed.Methods
MRI data were acquired at 3T on a Siemens Prisma system for seven suitably consenting patients suspected of having prostate cancer lesions based on their clinical history. For all except one of these patients, at least one of the detected lesions was assigned a PI-RADS score of at least 3 according to PI-RADS v2.1.1SPEN and EPI images were acquired at 2.4x2.4x3 mm3 nominal resolution. 32 slices were acquired for EPI; for SPEN this number was reduced to 12-16, which was sufficient to cover the whole prostate. EPI b-values (repetitions) of 50 (1), 500 (1), 900 (5) and 1400 (12) s/mm2 were acquired; SPEN’s corresponding b-values (repetitions) were 50 (2), 500 (2), 900 (5) and 1400 (10) s/mm2. In both cases images for four diffusion directions were collected. Hence, the total number of acquired DW images per slice for each modality was 76. For SPEN a reduced FOV along the PE direction was used (150x93 mm2, ROxPE), while FOV for EPI was 240x240 mm2. Corresponding TE’s were 52 and 68 ms for EPI and SPEN, respectively. SPEN’s longer TE vs EPI despite its acquisition of fewer lines along the PE direction reflects the additional delays necessary to fulfill SPEN’s full refocusing condition.10
The high number of “similar” images acquired per each slice, namely 76, motivated us to jointly reconstruct all the SPEN b-weighted images acquired per-slice, incorporating locally low rank (LLR) constraints8 (i.e., Eq. 1 in Ref. [9]) with block size of 10×8 voxels (RO×PE) and a regularization parameter 𝜆 = 0.1. LLR regularization was used without constraining signals to any subspace, as no prior knowledge of signal evolution as a function of the applied b-value was assumed (otherwise, due to application of relatively high b-values, this could have led to deviations from a monoexponential signal decay model). The joint processing of all DW images using LLR regularization motivated a slightly different choice of the number of repetitions collected in SPEN vs EPI for each b-value, as better results in the former’s reconstruction were obtained if higher SNR in the b=50 s/mm2 images was ensured. This gain in SNR due to the joint processing of all acquired images over processing each image separately has been discussed previously for healthy volunteers.9 In addition to acquiring DW images and calculating from them ADC maps, virtual images corresponding to b=2000 s/mm2 were generated from the computed ADC maps and the b=50 s/mm2 images both for SPEN and EPI, to assist with the lesion identification.
Results & Discussion
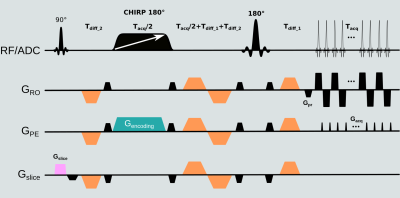
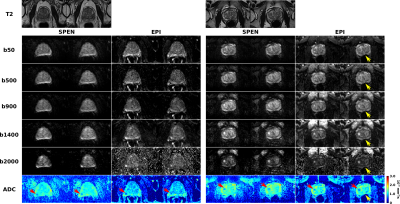
Figure 1 presents the SPEN sequence used in this study. In it, EPI’s linear PE is replaced with a quadratic PE achieved by a frequency swept adiabatic RF pulse applied in presence of a gradient, leading to a higher immunity to magnetic field inhomogeneities, as per the bandwidth of the chirped pulse.Figure 2 compares SPEN and EPI DWI in clinical context for two patients diagnosed with clinically significant lesions. While both modalities allow the detection of the lesions based on ADC maps and of calculated b=2000 s/mm2 images, SPEN allows to overcome some of the artifacts observed in EPI images (see Fig. 2, right panel). In addition, LLR regularized reconstruction endows SPEN ADC maps with either similar or better contrast –as illustrated for instance, in Fig. 2, left panel.
Conclusion
Results presented herein suggest SPEN could be an attractive alternative to single shot spin echo EPI for prostate DWI-based analyses, bringing both higher immunity to image artifacts arising from magnetic field inhomogeneities, as well better contrast –particularly perceptible in the ADC maps. Still, so far, all lesions identified in the SPEN images were also identified in EPI-based data.Acknowledgements
Support from the Minerva Foundation (Germany), the Israel Science Foundation (grants 2508/17 and 965/18), and the Clore Institute for Magnetic Resonance (Weizmann Institute), are acknowledged.References
(1) Turkbey, B.; Rosenkrantz, A. B.; Haider, M. A.; Padhani, A. R.; Villeirs, G.; Macura, K. J.; Tempany, C. M.; Choyke, P. L.; Cornud, F.; Margolis, D. J.; Thoeny, H. C.; Verma, S.; Barentsz, J.; Weinreb, J. C. Eur. Urol. 2019, 76, 340.
(2) Czarniecki, M.; Caglic, I.; Grist, J. T.; Gill, A. B.; Lorenc, K.; Slough, R. A.; Priest, A. N.; Barrett, T. Eur. J. Radiol. 2018, 102, 213.
(3) Hellms, S.; Gutberlet, M.; Peperhove, M. J.; Pertschy, S.; Henkenberens, C.; Peters, I.; Wacker, F.; Derlin, K. Medicine (Baltimore) 2019, 98, e16447.
(4) Barth, B. K.; Cornelius, A.; Nanz, D.; Eberli, D.; Donati, O. F. Invest. Radiol. 2015, 50, 785.
(5) Thian, Y. L.; Xie, W.; Porter, D. A.; Weileng Ang, B. Acad. Radiol. 2014, 21, 531.
(6) Tal, A.; Frydman, L. J. Magn. Reson. 2006, 182, 179.
(7) Ben-Eliezer, N.; Shrot, Y.; Frydman, L. Magn. Reson. Imaging 2010, 28, 77.
(8) Zhang, T.; Pauly, J. M.; Levesque, I. R. Magn. Reson. Med. 2015, 73, 655.
(9) Otikovs, M.; Basak, A.; Frydman, L. In Proceedings of the 2021 ISMRM and SMRT Annual Meeting and Exhibition; ISMRM, 2021, abstract number 732.
(10) Schmidt, R.; Frydman, L. Magn. Reson. Med. 2014, 71, 711.
Figures