2825
Assessing Skeletal Muscle Metabolism with PCr, NAD+NADH, and pH measurement with Dynamic 31P MRS at 7T1Department of Radiology, Brigham and Women's Hospital, Boston, MA, United States
Synopsis
A supine leg extension ergometer targeting the quadriceps femoris was built to be MR compatible and dynamic 31P-MR spectroscopy was acquired during an exercise challenge in a 7 Tesla MRI system. With the higher field strength, PCr and PCr recovery could be measured with a high confidence of fit without the need for additional smoothing and filtering steps. The increased spectral dispersion at 7T shows sufficient separation between the NAD+NADH resonance in addition to increased SNR. pH estimation in the active skeletal muscle was also performed.
Background
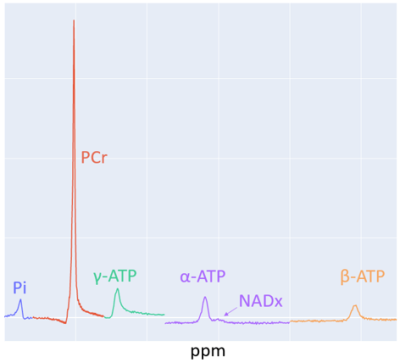
Deficiency in oxidative metabolism is associated with the pathophysiology of many conditions ranging from neurologic diseases1,2 to cardiovascular diseases3 in addition to liver4 and genetic dysfunction5. 31P MRS provides a noninvasive method of measuring metabolic markers such as Phosphocreatine (PCr), Inorganic Phosphate (Pi), Adenosine Triphosphate (ATP), etc. At 7T, additional metabolites such as Nicotinamide Adenine Dinucleotide it is oxidized and reduced form: NAD+/NADH can also be measured. Figure 1 shows an example of a 31P MR spectrum at 7T. This allows 31P MRS to reveal insights about cellular energy metabolism at the biochemical level in human subjects non-invasively6. In exercising skeletal muscle, dynamic 31P MRS can be used to obtain nearly real time measurements of phosphorous containing metabolites. One of the primary advantages of 31P MRS is that it is non-invasive and can therefore be used for treatment monitoring and to determine the effects of medical interventions. In this study we have quantified the aforementioned muscle metabolites and examined the consistency of these measures before and after an intervention.Methods
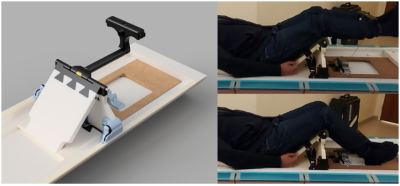
Ergometer Apparatus: A supine leg extension ergometer was designed and constructed specifically for performing exercise targeted at the quadriceps femoris muscle group within the bore of the magnet. The apparatus was constructed with plastic, composite material, and ceramic. The resistance is provided by Kevlar cables which connect the leg extension bar to lead weights outside the 200 Gauss line of the magnet (Figure 2).Acquisition and Exercise Protocol: All scans were acquired on a Siemens 7 T Terra MRI system with multinuclear capability and dual tuned 1H and 31P surface coil placed at the quadriceps femoris muscle. Proton images were acquired to ensure accurate placement. The 31P MRS protocol consisted of a localizer followed by non-localized pulse and acquire sequences (FID). After optimization of the 90 degree flip angle, a TR=12 s FID sequence was run while the subject remained at rest. Then, a TR=2 s FID was run that lasted the full 600 s duration of the exercise challenge protocol (120 s of rest, 180 s of exercise, and 300 s of rest). The subjects consisted of 35 subjects with a wide range of heights and weights. An intervention in the form of exercise or medication was given In all but seven subjects, a 31P MRS scan was acquired after the intervention such that a total of 63 scans were performed.
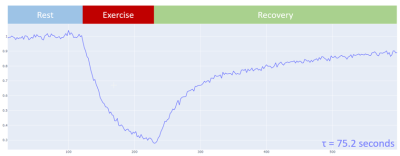
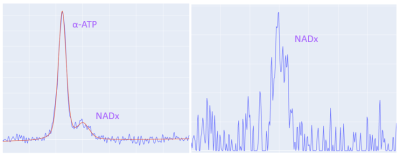
Data Processing and Analysis: The data was processed with a software package written in Python using standard libraries, numpy, and lmfit (for fitting resonances). The NAD+/NADH (NADx) combined resonance overlaps with the alpha-ATP resonance. This region of the spectrum requires a multipeak model to fit and separate the NADx signal, whereas a single peak fit is sufficient for the other resonances of interest. The PCr measurement from the short TR FID acquisition (TR=2 s) provides dynamic estimates through the exercise challenge. During the rest phase following exercise, the exponential PCr recovery is fit and the time constant, τ, of this fit is calculated. The τ value provides a singular value in units of seconds that can be used as a marker for the state of metabolic function in the skeletal muscle. The dynamic intracellular pH in exercising skeletal muscle cells is calculated from the ppm shift between PCr and Pi.
Results and Discussion
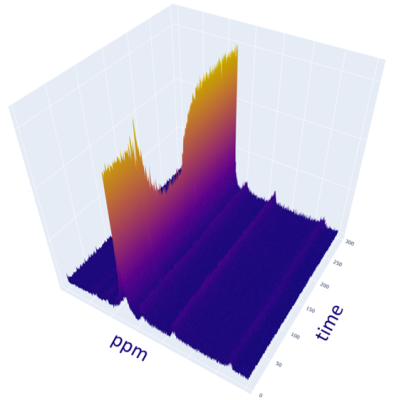
Figure 3 shows an example of the dynamic 31P MR Spectra acquired during exercise and Figure 4 shows the changing PCr signal over time. Estimate of PCr and PCr recovery with exponential fitting showed consistent results between the pre-intervention scan measurements and post-intervention scan measurements (pre: 62.4+24.9, post: 61.0+27.5). The high variability is due to intrasubject changes due to intervention or placebo to which the analysis was blinded as an ongoing study however there were no major outliers demonstrating reliable measures. More importantly, the average Root Mean Square Error (RMSE) of the exponential fit for estimating τ values was 0.0230 pre-intervention and 0.0216 post-intervention showing no significant difference.The NADx resonances were fit and separated from the adjacent alpha-ATP resonances as shown in Figure 5. The NADx peak area was normalized with the beta-ATP peak to obtain the NADx/beta-ATP ratio. The mean and standard deviation of the NADx/beta-ATP ratio was 0.217+ 0.187, before the intervention and 0.157+0.060, after.
Finally, the pH drop in skeletal muscle during the exercise was measured. Before the intervention the average minimum pH during exercise was 6.28 and after it was 6.22.
Conclusion
The results from the quantified values obtained the 31P MR Spectra show consistency before and after the intervention. This suggests that the 31P MRS acquisition protocol, exercise apparatus, and software pipeline a functioning as intended. Further data analysis from this study will utilize unblinded groups. Group differences between control subjects and subjects who received the intervention will be investigated. The preliminary results suggest 31P MRS can be a consistent and reproducible tool for measuring skeletal muscle energy metabolism.Acknowledgements
No acknowledgement found.References
1. Hu, M. T. M., et al. "Cortical dysfunction in non-demented Parkinson's disease patients: A combined 31P-MRS and 18FDG-PET study." Brain 123.2 (2000): 340-352.
2. Klunk, W. E., et al. "Quantitative 1H and 31P MRS of PCA extracts of postmortem Alzheimer's disease brain." Neurobiology of aging 17.3 (1996): 349-357.
3. Yabe, T. "Mitsunami K, Inubushi T, and Kinoshita M." Quantitative measurements of cardiac phosphorus metabolites in coronary artery disease by 31: 15-23.
4. Munakata, T., et al. "An in vivo 31P MRS study of patients with liver cirrhosis: progress towards a non‐invasive assessment of disease severity." NMR in Biomedicine 6.2 (1993): 168-172.
5. Vorgerd, M., and J. Zange. "Treatment of glycogenosys type V (McArdle disease) with creatine and ketogenic diet with clinical scores and with 31P-MRS on working leg muscle." Acta Myologica 26.1 (2007): 61.
6. Damon, Bruce. "P-31 magnetic resonance spectroscopy in skeletal muscle: Experts' consensus recommendations." (2020).
Figures