2823
Octopus rubescens as a model for the development and validation of muscle DTI tractography1Beckman Institute, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 2NeuroSpin, Gif-sur-Yvette, France, 3Department of Mechanical Science and Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States
Synopsis
We present data from a pilot study of an Octopus rubescens arm demonstrating it is possibly to identify the complex structural arrangement of its musculature using MRI and DTI tractography. All major muscle groups of the octopus arm are able to be identified and extracted using DTI tractography. Overall, this pilot study establishes cephalopod arms as an appealing testbed for the development of muscle MRI segmentation and tractography algorithms.
Introduction
Use of DTI tractography to extract the morphology of different muscle groups has been established, however, such techniques are often limited to larger muscles such as those in the lower limb.1 DTI of smaller and complex muscles such as paraspinal muscles2 or the tongue is more difficult.3 Further, tractography validation requires dissection and histology to establish the true arrangement of these muscles, which is difficult to perform in a way that preserves the in vivo arrangement of the muscle architecture.Considering these challenges, an animal model that allows testing and validation of muscle tractography algorithms is needed. Unique among large, complex organisms, cephalopods do not have bones, rather, their arms are arranged into a complex structure known as a muscular hydrostat.4 This combination of complex muscular arrangement and lack of rigid skeletons makes cephalopods an intriguing target for muscle tractography as they simultaneously present a difficult test for tractography algorithms while providing improved validation opportunities since histology can be performed with minimal deformation of the intrinsic muscle arrangement.
Here we perform a pilot study of an Octopus rubescens arm and establish that cephalopod arms are a viable model for investigating intricate muscular organization via MRI.
Methods
An Octopus rubescens was anesthetized in seawater with 333 nM MgCl concentration. An arm was amputated, fixed in 4% PFA for 48 hours and transferred to a 1x PBS solution. 24 hours prior to imaging the arm was placed in a tube with 15 mL of PBS and a 4 μL/mL gadolinium concentration. An arm was also removed from another specimen from which cross-sectional histology slices were obtained using H&E staining.T2-weighted and DTI scans were performed with a 9.4T Brucker preclinical scanner and a surface receive coil. Slice direction was aligned with the arm’s axial orientation. For T2-weighted scans, a TURBO RARE sequence was acquired over a 20 mm FOV with a 256x256 matrix (78.125 μm in-plane resolution), 20 slices (1 mm thickness), and TE/TR=32/2500 ms. DTI scans were acquired for a 128x128 matrix (156.25 μm resolution), 20 slices (1 mm thickness), TE/TR = 32/1500 ms, 4 segments, 3 b-values of 500, 1000, and 1500 mm2/s, 12 directions, and 16 averages. Fiber tractography was performed using DSIstudio for two ROIs.5 The first considered only the isolated central arm musculature while the second considered the tissue surrounding a single sucker.
Results
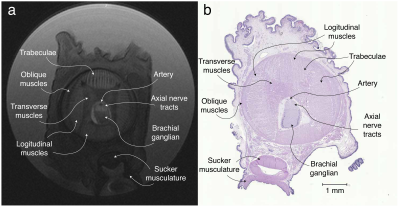
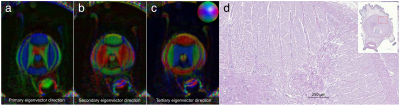
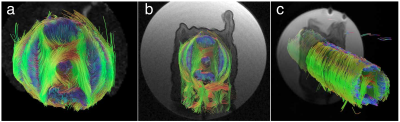
A cross-sectional T2-weighted image is shown in Fig 1 alongside the H&E-stained microscopy. Both clearly show the multiple known muscle groups of the octopus arm6 including longitudinal and transverse muscles as well as the trabeculae muscles that enervate the longitudinal muscles. The source of the observed contrast between muscle groups is unknown. In the arm’s center, the main artery and axial nerve cord are visible. Within the nerve cord, the two axial nerve tracts that run along the arm and the brachial ganglia, which is slanted towards the nearest sucker, are identifiable. Finally, the sucker’s musculature is visible, with the muscles that allow the sucker to generate negative pressure identifiable, however, lack of signal due to distance from the surface coil hampers analysis.Examination of DTI eigenvectors in Fig. 2 shows all three eigenvectors are spatially organized, which is potentially the effect of large-scale muscle organizatioin (e.g. trabeculae). Similar secondary and tertiary eigenvector organization has been observed in cardiac7 and skeletal muscle8,9,10. For tractography, inspection of the tractogram shows it self-organizes into a structure that strongly correlates with the muscle groups of the octopus (Fig. 3). The four longitudinal muscle groups, the transverse and oblique muscles, and the axial nerve cord are clearly observed. Expanding the tractography area, it appears possible to extract sucker morphology, although the small muscle size and poor SNR limit this accuracy.
Discussion
There are important differences between octopus and human/mammalian tissue to consider. Specifically, octopus muscle cells are smaller6 (10-20 μm diameter) than mammalian muscle (50-80 μm). Additionally, octopus muscles are obliquely striated4 compared with cross-striated skeletal muscle, though this structural difference is likely below the spatial scale DTI is sensitive to. Finally, octopus muscles do not terminate into tendons or aponeuroses.Despite these limitations, as a complex muscular structure containing multiple, tightly intertwined muscle groups, cephalopod arms hold great potential for the development of muscle tractography algorithms. The lack of bones makes histological validation straightforward, with limited dissection needed, thus preserving the intrinsic musculature of the arm and allowing validation of tractography algorithms. Further, co-registration of MRI images with histology is simplified as the existence of alternating suckers provides clear registration landmarks.
Finally, there are many unanswered questions regarding octopus muscular organization and neuromuscular control of their arms and suckers. Beyond their use in medical applications, MRI and tractography may also serve as a useful tool for biologists, biomechanics, and robotics who are seeking to understand locomotion, manipulation, and control strategies of a range of different creatures.
Conclusions
We have presented data from a pilot study of an Octopus rubescens arm demonstrating it is possible to identify the complex structural arrangement of its musculature using MRI and DTI tractography. Overall, this pilot study demonstrates cephalopod arms to be an appealing testbed for the development of muscle MRI segmentation and tractography algorithms.Acknowledgements
This work was supported by ONR MURI grant N00014-19-1-2373 and a Beckman Institute postdoctoral fellowship.References
[1] Bolsterlee, Bart, Arkiev D'Souza, and Robert D. Herbert. "Reliability and robustness of muscle architecture measurements obtained using diffusion tensor imaging with anatomically constrained tractography." Journal of biomechanics 86 (2019): 71-78.
[2] Klupp, Elisabeth, et al. "Paraspinal muscle DTI metrics predict muscle strength." Journal of Magnetic Resonance Imaging 50.3 (2019): 816-823.
[3] Gaige, Terry A., et al. "Three dimensional myoarchitecture of the human tongue determined in vivo by diffusion tensor imaging with tractography." Journal of Magnetic Resonance Imaging 26.3 (2007): 654-661.
[4] Kier, William M. "The musculature of coleoid cephalopod arms and tentacles." Frontiers in cell and developmental biology 4 (2016): 10.
[5] Yeh, F. C. "Diffusion MRI reconstruction in DSI studio." Advanced Biomedical MRI Lab, National Taiwan University Hospital. Available online at: http://dsi-studio.labsolver.org (2017).
[6] Kier, William M., and Michael P. Stella. "The arrangement and function of octopus arm musculature and connective tissue." Journal of morphology 268.10 (2007): 831-843.
[7] Dou, Jiangang, et al. "Combined diffusion and strain MRI reveals structure and function of human myocardial laminar sheets in vivo." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 50.1 (2003): 107-113.
[8] Galban, Craig J., et al. "Diffusive sensitivity to muscle architecture: a magnetic resonance diffusion tensor imaging study of the human calf." European journal of applied physiology 93.3 (2004): 253-262.
[9] Karampinos, Dimitrios C., et al. "Myofiber ellipticity as an explanation for transverse asymmetry of skeletal muscle diffusion MRI in vivo signal." Annals of biomedical engineering 37.12 (2009): 2532.
[10] Gharibans, A. A., et al. "Reconstruction of 3D fabric structure and fiber nets in skeletal muscle via in vivo DTI." International society for magnetic resonance in medicine, Montreal (2011): 1154.
Figures