2818
The effect of systematic errors reduction in the brain DTI on the 1.5T clinical MR scanner.1Faculty of Geology, Geophysics and Environmental Protection, AGH University of Science and Technology, Kraków, Poland, 2Faculty of Physics and Applied Computer Sciences, AGH University of Science and Technology, Kraków, Poland, 3Department of Diagnostic Imaging, Jagiellonian University Medical College, Kraków, Poland
Synopsis
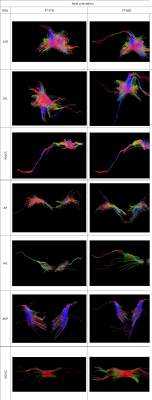
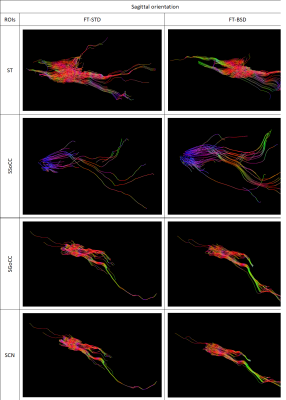
BSD DTI is a method to reduce systematic errors observed in standard Diffusion Tensor Imaging (DTI) of anisotropic tissues. In the work the effect of systematic errors reduction was shown based on DTI of the brain. Diffusion tensor parameters and tractography were compared between standard DTI (STD) and after the application of BSD DTI method in 11 ROIs chosen in axial and sagittal image planes. Depending on ROI eigenvalues and FA were under- or overestimated in STD, which was corrected by BSD DTI. Reduction of systematic errors leads to decreased standard deviation and improved diffusion tensor tractography.
Introduction
Diffusion tensor imaging (DTI) is a MRI technique used for the description of anisotropic diffusion in the brain to estimate the axonal (white matter) organization.[1] Brain can be visualized by a fiber tractography (FT), which is based on DTI and reflects neural tracts. The two main parameters derived from DTI describing differences between biological tissues microstructure are mean diffusivity (MD) and fractional anisotropy (FA).[2] FA reflects the degree of molecular displacement anisotropy and varies between 0 (isotropic diffusion) and 1 (infinite anisotropic diffusion). MD reflects the average magnitude of diffusive molecular displacement and can be directly related to the medium size and anisotropy.[3] Although MD is similar in the grey and white matter in adult human brain, FA is very different in these tissues as a result of their unique structure. Anisotropy can facilitate early detection of brain tissues pathology, such as the assessment of the white matter deformation, delineation of the anatomy of immature brains[4], presurgical planning [5], early detection of neurodegenerative diseases [6]. It can also be a source of information about brain regions asymmetry, sex-[7] and age-related[8] degree of myelination during neurodevelopment and microstructural changes reflecting axonal degeneration. However, quantitative evaluation of the above issues through DTI requires a high experimental and calculative accuracy. Common concerns are related to the impact of noise and systematic errors on the obtained diffusion tensor parameters. Magnetic field is assumed homogeneous for the applications in clinical practice, while multiple research showed magnetic field and gradient inhomogeneity and nonlinearity. This leads to significant systematic errors in the obtained DTI parameters. Their elimination proved to be effective through the appropriate calibration by b-matrix spatial distribution method (BSD-DTI), which is based on gradient inhomogeneity correction in all directions using phantoms. [9, 10] BSD-DTI can help to define FA and MD more precisely and improve the accuracy of fiber tracts [1, 2] . The aim of this study was to reveal the impact of systematic errors on the DTI parameters and tractography in the several human brain areas. This was achieved via the assessment of the effect of BSD-DTI and in comparison to the standard (i.e. without calibration) approach (STD).Methods
DTI measurements of the brain of a 46-year-old volunteer were conducted on a 1.5 T system Siemens Avanto FIT (Elrangen, Germany) equipped with dedicated 32 channel head coil, with 33 mT/m gradient strength. The SE-EPI sequence was used with the following parameters: b-values of 0 and 1000 s/mm2, 6 diffusion gradient directions, echo time, TE= 105 ms, repetition time, TR= 9.5 s, 192x192 matrix and slice thickness of 3mm. The components of the diffusion tensor and fiber tracts for the selected ROIs were calculated by the STD and BSD-DTI approaches. The obtained data were post-processed applying a home-build BSD-DTI 2.0 software (NMR LaTiS, AGH-UST Krakow). The selected ROIs were collected in white matter (WM) and grey matter (GM). WM ROIs are localized in the axial and sagittal planes and encompassed: Genu (AGoCC) and Splenium (ASoCC) of Corpus Callosum, Internal Capsule (AIC). GM ROIs located in sagittal plane are Genu (SGoCC) and Splenium (SSoCC) of Corpus Callosum,Thalamus (ST) and Caudate Nucleus (SCN), while Thalamus (ATL, ATR), Putamen (AP) and Globus Pallidus (AGP) are in the axial one.Results
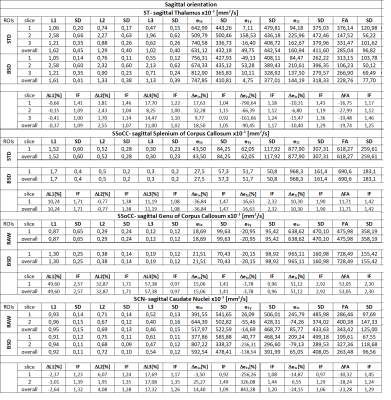
The greatest alteration of FA was observed in first slice of Genu of Corpus Callosum. In Genu of Corpus Callosum we can observe the highest values of every DTI parameter and the greatest effect of BSD-DTI especially on FA. The value of FA in STD and BSD-DTI was 0.57874 and 0.75384, respectively. The improvement factor (IF; the ratio of standard deviations (SDs) from STD and BSD-DTI) of this ROI was equal 3.78. In contrary, the lowest IF of FA was observed in the third slice of axially oriented leftward Thalamus, which was equal to 1.08. However, FA values in this region obtained by STD and BSD-DTI were visibly different and equal to 0.45412 and 0.37250. Details of BSD-DTI effect in all ROIs is shown in Table 1 and 2.Discussion
Application of BSD-DTI evidently has an impact FA value in the brain. Significant changes are also seen for eigenvalues and the first eigenvector. It also decrease SD value in each case. Qualitative evaluation of tractographies indicate that fiber tracts are improved in most cases in terms of density, length and direction.Conclusions
We showed the effect of BSD-DTI method on the determined DTI parameters and tractography in WM and GM. Our study shows that reduction of systematic errors is essential for the accurate quantitative characterization of the brain.Acknowledgements
Examination was financed by Medical Research Agency contract no: 2020/ABM/01/00006-00.References
[1] S. Wakana, A. Caprihan, M. M. Panzenboeck, J. H. Fallon, M. Perry, R.L. Gollub, K. Hua, J. Zhang, H. Jiang, P. Dubey, A. Blitz, Peter van Zijl, and S. Moria “Reproducibility of quantitative tractography methods applied tocerebral white matter.” NeuroImage (2007); 36: 630- 644.
[2] Basser, P.J. & Pierpaoli, C. “Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI.” Journal of Magnetic Resonance (1996), Series B; 111: 209-219.
[3] Özarslan, E. Vemuri, B.C. & Mareci, T. H. “Generalized scalar measures for diffusion MRI using trace, variance, and entropy.” Magnetic Resonance in Medicine (2005), 53: 866-876.
[4] Lindsay Snook, Lori-Anne Paulson, Dawne Roy, Linda Phillips, and Christian Beaulieu “Diffusion tensor imaging of neurodevelopment in children and young adults.” NeuroImage (2005) 26: 1164 – 1173.
[5] Qiu, T., Zhang, Y., Wu, J.-S., Tang, W.-J., Zhao, Y., Pan, Z.-G. “Virtual reality presurgical planning for cerebral gliomas adjacent to motor pathways in an integrated 3-D stereoscopic visualization of structural MRI and DTI tractography. “ Acta Neurochirurgica (2010)., 152(11): 1847–1857.
[6] Hans-Peter Müller and Jan Kassubek “Diffusion Tensor Magnetic Resonance Imaging in the Analysis of Neurodegenerative Diseases.” Journal of Visualizes Experiments (2013) 77.
[7] Owen R. Phillips, Kristi A. Clark, Eileen Luders, Ramin Azhir, Shantanu H. Joshi, Roger P. Woods, John C. Mazziotta, Arthur W. Toga and Katherine L. Narr “Superficial White Matter: Effects of Age, Sex, and Hemisphere.” BRAIN CONNECTIVITY (2013), Volume 3, Number 2.
[8] Khader M. Hasana,, Amal Iftikhara, Arash Kamalia, Larry A. Kramera, Manzar Ashtarie, Paul T. Cirinoc,d, Andrew C. Papanicolaoub, Jack M. Fletcherc, Linda Ewing-Cobbsb “Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography.” Brain Research (2009), 1276: 67-76.
[9] Borkowski K, Krzyżak AT. Analysis and correction of errors in DTI-based tractography due to diffusion gradient inhomogeneity. J Magn Reson. (2018 Nov); 296: 5-11.
[10] R, Obuchowicz, A. Krzyżak “Improvement of brain tractography using the BSD-DTI method on the example of subcortical u-fibers visualisation.” (2019).
[11] K. Kłodowski and A. T. Krzyżak, "Innovative anisotropic phantoms for calibration of diffusion tensor imaging sequences," Magnetic Resonance Imaging (2016 May), vol. 34, no. 4: pp. 404-409.
Figures