2816
Image-based computer simulations of haemodynamics and blood cells distributions: A comparison between MRI and ultrasound1School of Engineering, University of Warwick, Coventry, United Kingdom, 2School of Psychology and Clinical Language Sciences, University of Reading, Reading, United Kingdom, 3University of Herefordshire, Hatfield, United Kingdom, 4Department of Radiology, Royal Berkshire Hospital, Reading, United Kingdom, 5Medical School, University of Warwick, Coventry, United Kingdom
Synopsis
MRI-based CFD simulations are compared with ultrasound-based CFD to ascertain the capability of ultrasound.
Introduction
Image-based computational fluid dynamics (CFD) simulations have been widely used to understand the underlying haemodynamic characteristics. Different imaging modalities including computed tomography (CT)1 and magnetic resonance imaging (MRI)2 have been used in previous CFD simulations while other techniques such as ultrasound scanning3, digital subtraction angiography or X-ray4 and 3D rotational angiography imaging5 are also clinically available. While ultrasound imaging is ideal for repeat scans, it can suffer from imaging artefacts and has yet to be thoroughly validated for use in CFD simulation6. In this study, MRI-based CFD simulation results are compared with the ultrasound-based CFD to assess the capability of ultrasound-based CFD in predicting hemodynamic quantities.Methods
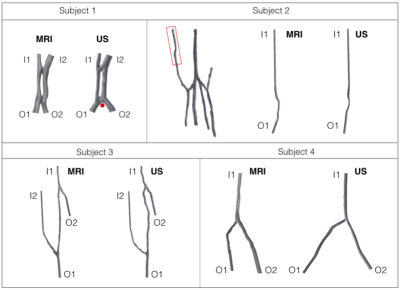
Four healthy subjects were recruited for this study by the Royal Berkshire Hospital, UK. Their forearm veins including the cephalic vein were reconstructed from MRI and ultrasound images of their right forearms. MRI images were acquired using an optimised time-of-flight scan (TR=20ms, TE=4.57ms, FoV=250mm, flip angle=30, GRAPPA: acceleration factor of 2), using a 4-channel large-flexi coil wrapped around the arm in line with the bottom of the wrist. To improve the definition of vasculature, an MR-compatible tourniquet was applied, and the subjects were scanned with their arm raised above the head towards the centre of the bore. 3D US images were acquired using consecutive 2D image slices aligned along the axial direction. The time of flight for ultrasound scan is approximately 15 seconds over a distance of approximately 250mm. Figure 1 shows four pairs of reconstructed MRI and their corresponding ultrasound models for each subject with the distal side at the top. This geometrical difference, and the vessel tortuosity observed in the ultrasound model of subject 3, is often encountered as a result of using hand-held ultrasound transducer.All reconstructed models from MRI and ultrasound images were spatially discretised in Star-CCM+ (Siemens) and used for CFD simulations. A pulsatile mass flow rate derived from an appropriate patient-specific velocity waveform7 was prescribed at each inlet. Constant pressure was imposed to all outlets8 where the cross-sections were extended by 10 times the respective diameter to prevent downstream perturbation9. The first three cardiac cycles showing transient flow behaviour were discarded and results from the fourth cardiac cycle were analysed to ensure temporal periodicity.
Results
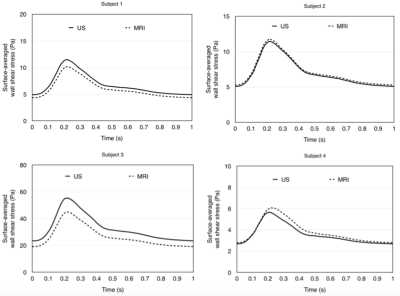
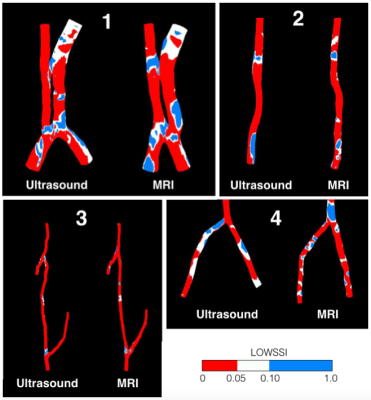
Haemodynamic quantities between MRI and ultrasound models for all four subjects are compared in this section. Volumetric flow rates predicted by the ultrasound models were compared with those predicted by MRI. Temporal variations of mass flow rates over a cardiac cycle can be observed. The MRI-US differences for all four subjects, except for subject 1, are well below 20%, indicating a good agreement between the two models.Surface-averaged wall shear stress (SAWSS) was measured and its temporal variations for MRI and US models are compared Figure 2. There are no significant MRI-US differences in all models, except for subject 3 where the percentage difference slightly exceeds 20%. The variations of SAWSS throughout a cardiac cycle are shown in Figure 2. In the present study, further comparisons between MRI and US-based CFD simulations have been made with other WSS parameters. Various endothelial changes relevant to stenotic development reported in literature are compared between MRI and ultrasound CFD. Comparisons of these wall shear stress quantities allow the capability of ultrasound in discriminating healthy regions from areas prone to stenotic development to be validated against MRI. As shown in figure 3, both US and MRI correctly predict that the majority of the surface area corresponds to very low LOWSSI (< 0.1). TAWSS, OSI, LOWSSI, and WSS duty factors (DFs) measured by MRI and ultrasound are further compared.
Discussion
While most previous CFD studies used MRI or CT models for blood vessels reconstruction, only a limited number of studies used ultrasound models mainly due to the moderate loss of vessel curvature and excessive imaging artifacts involved. To the best of the authors’ knowledge, no previous studies have investigated whether non-invasive ultrasound scanning can replace the already well-established MRI in predicting haemodynamics and blood cells distribution in a non-Newtonian blood vessels. The present study has shown that ultrasound-derived CFD results are not significantly different from those derived from MRI-based CFD simulations.This study is a precursor to arteriorvenous fistula CFD study. As fistula maturation is closely related to the blood flow dynamics, image-based CFD simulations can inform the underlying haemodynamic characteristics in a fistula and thus explain the success or failure of fistula maturation10.
Conclusion
This MRI-US comparison study suggests that ultrasound-based CFD is capable of predicting subject specific haemodynamics that is comparable to those derived from MRI. Additionally, the proportions of blood vessel walls experiencing low and oscillating wall shear stress predicted by both modalities presented no systematic bias and correctly confirm the low possibility of intimal hyperplasia (IH) of these healthy blood vessels. This demonstrates the capability of US-based CFD in predicting haemodynamic quantities of healthy subjects as well as intimal hyperplasia due to high wall shear stress, which is useful in evaluating haemodialysis patency of AV fistulas.Acknowledgements
The financial support from the Joint Academic Board of University of Reading and Royal Berkshire Hospital is greatly appreciated. The authors acknowledge the use of HPC facilities at Scientific Computing RTP, University of Warwick.References
1. L. Grechy, F. Iori, R. W. Corbett, S. Shurey, W. Gedroyc, N. Duncan, C. G. Caro, and P. E. Vincent. Suppressing unsteady flow in arteriovenous fistulae. Physics of Fluids, 29(10): 101901, 2017.
2. M. Sigovan, V. Rayz, W. Gasper, H. F. Alley, C. D. Owens, and D. Saloner. Vascular remodeling in autogenous arteriovenous fistulas by MRI and CFD. Annals of Biomedical Engineering, 41(4):657–668, 2013.
3. P. M. McGah, D. F. Leotta, K. W. Beach, and A. Aliseda. Effects of wall distensibility in hemodynamic simulations of an arteriovenous fistula. Biomechanics and Modeling in Mechanobiology, 13(3):679–695, 2014.
4. B. Ene-Iordache, L. Mosconi, G. Remuzzi, and A. Remuzzi. Computational fluid dynamics of a vascular access case for hemodialysis. Journal of Biomechanical Engineering-Transactions of the ASME, 123(3):284–292, 2001.
5. H. G. Morales, O. Bonnefous, A. J. Geers, O. Brina, V. M. Pereira, L. Spelle, J. Moret, and I. Larrabide. Does arterial flow rate affect the assessment of flow-diverter stent performance? American Journal of Neuroradiology, 37 (12):2293–2298, 2016.
6. A. Gee, R. Prager, G. Treece, and L. Berman. Engineering a freehand 3d ultrasound system. Pattern Recognition Letters, 24(4-5):757–777, 2003.
7. B. Ene-Iordache and A. Remuzzi. Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: low and oscillating shear stress locates the sites of stenosis. Nephrology Dialysis Transplantation, 27(1):358–368, 2012.
8. P. M. McGah, D. F. Leotta, K. W. Beach, J. J. Riley, and A. Aliseda. A longitudinal study of remodeling in a revised peripheral artery bypass graft using 3d ultrasound imaging and computational hemodynamics. ASME Journal of Biomechanical Engineering, 133(4):10, 2011.
9. T. Otani, T. Shindo, S. Li, M. Hirata, and S. Wada. Effect of Local Coil Density on Blood Flow Stagnation in Densely Coiled Cerebral Aneurysms: A Computational Study Using a Cartesian Grid Method. J Biomech Eng, 140(4), Apr 2018.
10. J. E. Carroll, E. S. Colley, S. D. Thomas, R. L. Varcoe, A. Simmons, and T. J. Barber. Tracking geometric and hemodynamic alterations of an arteriovenous fistula through patient-specific modelling. Computer Methods and Programs in Biomedicine, 186: 105203, 2020.
Figures