2807
Evaluation of the REFILL dynamic distortion correction method for fMRI1Department of Neurology, Medical University of Graz, Graz, Austria, 2Centre for Advanced Imaging, University of Queensland, Brisbane, Australia, 3Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 4UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 5School of Electrical Engineering and Information Technology, University of Queensland, Brisbane, Australia, 6Siemens Healthineers, Brisbane, Australia
Synopsis
We evaluate the performance of a recently-proposed dynamic distortion correction (DDC) method for fMRI at 7T, with a task which generates field fluctuations. The Reverse-Encoded First Image and Low resoLution reference scan (REFILL) method generates fieldmaps from the phase of standard, single-echo EPI from fMRI time series, using coil sensitivity information from a fast reference scan and removing other, non-B0-related contributions to the phase, which are calculated from a readout-reverse EPI volume. In contrast to conventional static distortion correction (SDC) with a GE-based fieldmap, REFILL captured dynamic changes to the field, leading to an accurate correction and increased tSNR.
Introduction
A map of variations in the static magnetic field, ΔB0, derived from the phase of a multi-echo reference scan, can be used to calculate and remove susceptibility-related image distortions in EPI1, but this static distortion correction (SDC) does not capture changes in ΔB0 due to system instabilities, motion and breathing. In Dynamic Distortion Correction (DDC), a fieldmap is derived from the phase of each EPI volume in the fMRI time series2,3,4,5. This offers the potential for a more robust correction, but many DDC methods require multi-echo EPI, time-consuming reference scans and/or complex image processing. A DDC method for single-echo EPI called REFILL (Reverse-Encoded First Image and Low resoLution reference scan) was proposed recently. It improves on previous DDC approaches by i) reducing the time for the required reference data to a few seconds ii) removing the need to unwrap the reference data and iii) introducing a correction for non-B0-related phase specific to EPI6. We compare the performance of REFILL DDC, in vivo and at 7T, with two SDC methods - 'gold standard' GE-based fieldmapping (GE-SDC) and the popular TOPUP7 method (TOPUP-SDC) - and evaluate the performance of REFILL in an fMRI experiment involving dynamic fluctuations in ΔB0.Materials and Methods
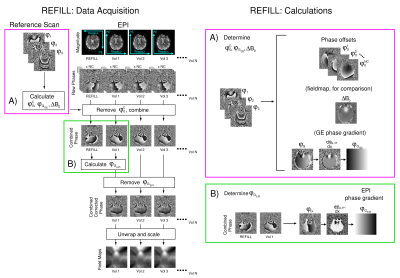
In REFILL, a dynamic series of fieldmaps $$$\Delta B_{0,t}$$$ is calculated as follows: $$\Delta B_{0,t}=\frac{1}{\gamma \cdot TE_{EPI}}\angle\sum^c \left(M^c_t\right)^2 \cdot \exp [i\cdot\left({\theta^c_t-\theta^c_0 + \phi_{G_{EPI}}}\right)+n2\pi],$$where $$$M^c_{t}$$$ is the magnitude of the image from coil $$$c$$$ in the tth image in the time series, and $$$\theta^c_t$$$ the phase, $$$\theta^c_0$$$ is the phase offset (which is calculated from the multi-echo reference scan) and $$$\phi_{G_{EPI}}$$$ is the phase gradient in the EPI time series in the readout direction, which is calculated from the readout-reversed first image (see Fig.1).Data from three healthy subjects were acquired with a Siemens 7T+ scanner with software VE12U_AP01 (Siemens Healthcare, Erlangen, Germany) and a 32-channel head coil (Nova Medical, MA, USA); a low resolution bipolar 2D MGE reference scan (64*64 matrix, 40 slices, TE={2.5,5.0,7.5}ms, GRAPPA=2, TA=9s), followed by a conventional fieldmap for the comparison GE-SDC approach (128*128 matrix, 40 slices, TE={2.5,5.0,7.5}ms, GRAPPA=2, TA=14s). After 1 EPI volume in which the phase-encode (PE) and readout directions were reversed (for both TOPUP-SDC and calculation of $$$\phi_{G_{EPI}}$$$ in REFILL), 100 volumes of 2D EPI (PE=PA, 128*128*40 slices, GRAPPA=2, TE/TR=22/2270 ms) were acquired, during which subjects performed a self-paced motion of the hands to and from the coil (circa 10s period), causing changes to $$$\Delta B_{0,t}$$$. Phase offsets were calculated from the reference scan with ASPIRE8 and removed from EPI, prior to channel combination, in ICE. Offline, fieldmaps were calculated by unwrapping phase images with ROMEO9, masking using phase temporal coherence9, and interpolation using the smoothn.m function in MATLAB.
Results
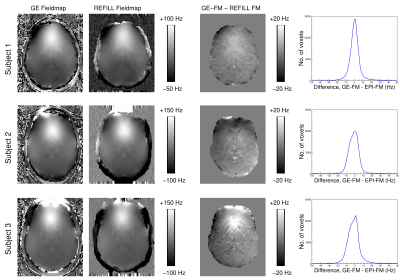
There was good agreement between EPI-based REFILL fieldmaps and GE-based fieldmaps (Fig.2) acquired in immediate succession (i.e. with minimal motion), with average 10th and 90th percentiles of the difference being -7.8±1.9 Hz and 7.5±0.5Hz respectively, over subjects.REFILL fieldmaps agreed with reference GE fieldmaps to a much better degree than TOPUP fieldmaps (Fig.3). With TOPUP, the disparity in field estimates was up to 50 Hz over substantial regions of the brain, with fieldmap errors of high spatial frequency (red arrow) leading to a shadowing of the ventricles in the corrected image (yellow arrows), and residual distortions in correction of the “distortion field” (bottom row, red circles). The REFILL fieldmap agreed with the GE reference fieldmap to within a few Hz over most of the image with significant deviations only at low signal regions close to the edge of the image (blue arrows), which did not affect the quality of the correction (see e.g. position of the ventricles in the zoomed image).
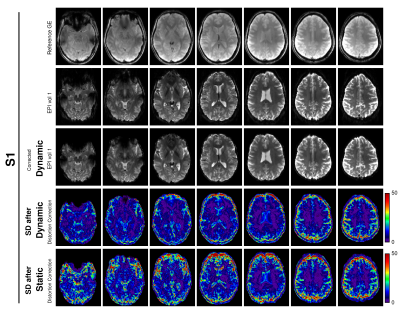
In the case of a dynamically changing field, fluctuating distortions remained in the images corrected with GE-SDC. These led to high standard deviations of voxel values over time, particularly at high contrast boundaries (Fig.4). The standard deviation was much lower in REFILL-corrected time series, and most dynamic distortions within the brain were removed (same figure) – albeit with some residual fluctuations at the edge of the brain. The differences between the two approaches are best visualized in movies for the 3 subjects (S1:http://alturl.com/2bmge, S2:http://alturl.com/tx362 and S3:http://alturl.com/z6w5w).
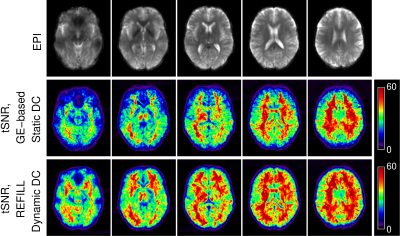
The mean tSNR over subjects was substantially higher in images corrected with REFILL DDC than with SDC (Fig.5).
Discussion and Conclusion
In the absence of a deliberate change in shim, single-echo fieldmaps from the REFILL dynamic distortion correction method agreed well with the static GE-based fieldmap and were more accurate than the fieldmap generated by TOPUP. In an experiment in which the field changed dynamically, REFILL provided a more accurate correction, reducing the standard deviation of the time series and increasing the tSNR compared to SDC.REFILL requires just one, low-resolution bipolar triple-echo reference scan (TA circa 5-10s) and one EPI volume with reversed readout direction. There is no need for phase unwrapping in the online calculation, making it possible to generate phase images which reflect only local variation in B0 on the scanner reconstructor. These features make it feasible to apply REFILL routinely with a wide range of EPI sequences used for fMRI (e.g. simultaneous multi-slice, 3D-EPI), without the need for sequence modifications.
Acknowledgements
This study was funded by the Marie Skłodowska-Curie Action MS-fMRI-QSM 794298. Additional support from the Austrian Science Fund (FWF): 31452 and the Austrian Federal Ministry for Digital and Economic Affairs and the National Foundation for Research, Technology and Development is gratefully acknowledged. SB and MB acknowledge funding from the NHMRC-NIH BRAIN Initiative Collaborative Research Grant APP1117020, NIH grant 1R01MH111419. The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Centre for Advanced Imaging, the University of Queensland.References
1. Jezzard P. Correction of geometric distortion in fMRI data. NeuroImage 2012;62:648–651 doi: 10.1016/j.neuroimage.2011.09.010.
2. Visser E, Poser BA, Barth M, Zwiers MP. Reference-free unwarping of EPI data using dynamic off-resonance correction with multiecho acquisition (DOCMA). Magnetic Resonance in Medicine 2012;68:1247–1254 doi: https://doi.org/10.1002/mrm.24119.
3. Marques JP, Bowtell R. Evaluation of a new method to correct the effects of motion-induced B0-field variation during fMRI. Proceedings of the 13th meeting of the ISMRM 2005:510.
4. Dymerska B, Poser BA, Barth M, Trattnig S, Robinson SD. A method for the dynamic correction of B 0 -related distortions in single-echo EPI at 7 T. NeuroImage 2018;168:321–331 doi: 10.1016/j.neuroimage.2016.07.009.
5. Lamberton F, Delcroix N, Grenier D, Mazoyer B, Joliot M. A new EPI-based dynamic field mapping method: application to retrospective geometrical distortion corrections. J Magn Reson Imaging 2007;26:747–55.
6. Robinson S, Bachrata B, Eckstein K, Trattnig S, Enzinger, Christian, Barth, Markus. Improved dynamic distortion correction for fMRI using single-echo EPI, a fast sensitivity scan and readout-reversed first image (REFILL). Proceedings of the 2021 ISMRM & SMRT Annual Meeting & Exhibition (Virtual) 2021:671.
7. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 2003;20:870–888 doi: 10.1016/S1053-8119(03)00336-7.
8. Eckstein K, Dymerska B, Bachrata B, et al. Computationally Efficient Combination of Multi-channel Phase Data From Multi-echo Acquisitions (ASPIRE): Combination of Multi-Channel Phase Data from Multi-Echo Acquisitions (ASPIRE). Magn. Reson. Med 2018;79:2996–3006 doi: 10.1002/mrm.26963.
9. Dymerska B, Eckstein K, Bachrata B, et al. Phase unwrapping with a rapid opensource minimum spanning tree algorithm (ROMEO). Magnetic Resonance in Medicine 2021;85:2294–2308 doi: 10.1002/mrm.28563.
Figures