2793
Reduced field-of-view multi-shell DWI of the sciatic nerve: A reproducibility assessment
Ratthaporn Boonsuth1, Rebecca S Samson1, Francesco Grussu1,2, Marco Battiston1, Torben Schneider3, Masami Yoneyama4, Ferran Prados1,5,6, Carmen Tur1,7, Sara Collorone1, Rosa Cortese1, Claudia AM Gandini Wheeler-Kingshott1,8,9, and Marios C Yiannakas1
1NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 2Radiomics Group, Vall d’Hebron Institute of Oncology, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 3Philips Healthcare, Guildford, Surrey, United Kingdom, 4Philips Japan, Minatoku, Tokyo, Japan, 5Centre for Medical Image Computing, Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 6E-Heath Centre, Universitat Oberta de Catalunya, Barcelona, Spain, 7Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron Institute of Research, Vall d'Hebron Barcelona Hospital Campus, Barcelona, Spain, 8Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy, 9Brain Connectivity Research Centre, IRCCS Mondino Foundation, Pavia, Italy
1NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 2Radiomics Group, Vall d’Hebron Institute of Oncology, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 3Philips Healthcare, Guildford, Surrey, United Kingdom, 4Philips Japan, Minatoku, Tokyo, Japan, 5Centre for Medical Image Computing, Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 6E-Heath Centre, Universitat Oberta de Catalunya, Barcelona, Spain, 7Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron Institute of Research, Vall d'Hebron Barcelona Hospital Campus, Barcelona, Spain, 8Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy, 9Brain Connectivity Research Centre, IRCCS Mondino Foundation, Pavia, Italy
Synopsis
Advanced diffusion-weighted imaging (DWI) methods are powerful diagnostic and research tools when applied in the central nervous system (CNS). However, the use of DWI methods to study individual nerves outside the CNS in vivo, such as the sciatic nerve, has been hampered by a number of technical challenges. In this study, we explore the feasibility of acquiring multi-shell DWI metrics in the sciatic nerve using reduced field-of-view echo planar imaging and report results from a reproducibility assessment in in healthy controls.
Introduction
Diffusion-weighted imaging (DWI) can be used to characterise neural tissue composition, organization, and structure by providing both intra- and extra-cellular compartments information through sensitivity to Brownian motion of water molecules1,2. Most DWI studies have used single b-value diffusion acquisitions (“single shell”) and applied the diffusion tensor imaging (DTI) model to characterise diffusion patterns3. A new generation of models have been developed to leverage multiple b-values (“multi-shell”), which offer promising advances in characterising neural tissue in vivo. Measurements performed at varying b-values can be used to model more detailed features of the cellular environment4. A common multi-shell DWI method is diffusion kurtosis imaging (DKI), which aims to quantify the non-Gaussian characteristics of water diffusion within a voxel. Non-Gaussian diffusion is a hallmark of diffusion restriction or of the presence of several, distinct, Gaussian water pools. Its quantification in the context of peripheral nervous system (PNS) imaging is promising to assess microstructure, composition and structural organisation outside the CNS. Importantly, techniques such as DWI should provide metrics that are quantitative by construction, and thus be reproducible. However, there are many concerns in the context of longitudinal studies about reliability, repeatability and reproducibility of such quantitative measurements2,5. In this study, we explore the feasibility of acquiring multi-shell DWI metrics in the sciatic nerve using reduced field-of-view echo-planar imaging (ZOOM-EPI) as a new approach to alleviate some of the common technical problems associated with conventional single-shot EPI, and we report the assessment of the reproducibility in healthy controls at a single site.Methods
Participants: Eleven healthy controls (HC) (mean age 33.6 years, 7 female, range 24-50) were recruited with five out of eleven HCs scanned twice on separate occasions.MR imaging: A 3T Philips Ingenia CX system was used with the product SENSE spine and torso coils. In all HCs, both sciatic nerves were first located using the 3D ‘nerve-SHeath signal increased with INKed rest-tissue RARE Imaging’ (SHINKEI) sequence acquired in the coronal plane with a large FOV6,7 (Figure 1A). The parameters were as follows: TR=2200ms, TE=180ms, FOV=300×420mm2, voxel size=1.2×1.2×2mm3, NEX=1, TSE factor=56, improved motion-sensitized driven-equilibrium (iMSDE) duration=50ms, 170slices, scanning time=05:43min. The 3D SHINKEI acquisition was used to facilitate planning of subsequent scans on the right sciatic nerve: 1) a high-resolution fat-suppressed T2-weighted acquisition for segmentation of the sciatic nerve and 2) a ZOOM-EPI acquisition for calculation of the DWI metrics. The parameters for the T2-weighted acquisition were as follows: TR=5000ms, TE=60ms, FOV=180×180mm2, voxel size=0.5×0.5×5mm3, NEX=1, TSE factor=11, 30 slices, scanning time=08:08min. The multi-shell DWI acquisition was planned with identical scan geometry to the T2-weighted acquisition as follows: TR=6300 ms, TE=66 ms, FOV=64×48 mm2; voxel size=1×1×10 mm3; number of averages=2; half-scan=0.6; 12 slices; b=1000 s/mm2, 22 directions; b=2000s/mm2, 21 directions; b=3000s/mm2, 21 directions; 4 interleaved non-diffusion-weighted (b=0) images were also acquired; scanning time=17:31min.
Image analysis: Image segmentation of the sciatic nerve was manually performed in FSLview using the fat-suppressed T2-weighted image (Figures 1B and 1C). All DWI images were registered to the fat-suppressed T2-weighted image using affine registration with NiftyReg8. The DKI signal was fitted to the acquired multi-shell DWI data using the DiPy dkifit9,10 command; the fitting provided diffusion tensor metrics axial/radial/mean diffusivity (AD/RD/MD), fractional anisotropy (FA) and DKI metrics axial/radial/mean kurtosis (AK/RK/MK).
Statistical analysis: For the assessment of reproducibility, the coefficient of variation (%COV)11 was used to provide an estimate of the total amount of variability, with respect to the mean population value of the subject.
Results
An example of the FA map calculated in the sciatic nerve is shown in Figure 2. Figure 3 shows an example of the diffusion tensor metrics (AD/RD/MD), DKI metrics (AK/RK/MK) and fractional anisotropy (FA) from a single healthy control. In eleven HCs, mean (SD) MK, AK, RK, AD, RD, MD and FA in sciatic nerve were 0.95 (0.18), 0.79 (0.16), 1.10 (0.23), 2.06 (0.39) µm2 ms-1, 0.98 (0.23) µm2 ms-1, 1.35 (0.24) µm2 ms-1 and 0.41 (0.10) respectively. Table 1 shows the results of all DWI metrics calculated in all study participants. Table 2. shows reproducibility results expressed as the %COV.Discussion and Conclusion
In this study, we have explored the feasibility of obtaining multi-shell DWI metrics in the sciatic nerve using ZOOM-EPI by calculating diffusion tensor metrics (AD, RD and MD), DKI metrics (AK, RK and MK) and fractional anisotropy (FA) in eleven HCs. Moreover, we report the assessment of the reproducibility of these measurements by performing a comparison of sciatic nerve diffusion metrics from repeated measurements in five HCs, and by calculating the %COV. This pilot study confirms that both DTI imaging and DKI metrics can be measured with similar reproducibility for the multi-shell DWI technique of the sciatic nerve. Interestingly, greater variability in terms of reproducibility is found in FA (COV of 29.51%). We speculate that this finding may be due, at least in part, to high biological variability of FA, since our COV provides a measure of total variability without disentangling its biological and error-induced components. Further investigation is warranted to clarify this aspect using a larger sample size, which will be required to confirm the results presented here and more accurately quantify the reproducibility of DWI and DKI measurements in the sciatic nerve.Acknowledgements
The UK MS Society and the UCL-UCLH Biomedical Research Centre for ongoing support. CGWK receives funding from the MS Society (#77), Wings for Life (#169111), BRC (#BRC704/CAP/CGW), UCL Global Challenges Research Fund (GCRF), MRC (#MR/S026088/1), Ataxia UK. FP had a non-clinical Postdoctoral Guarantors of Brain fellowship (2017-2020). FP is supported by the National Institute for Health Research, UCL Hospitals Biomedical Research Centre. CT is being funded by a Junior Leader La Caixa Fellowship (fellowship code is LCF/BQ/PI20/11760008), awarded by “la Caixa” Foundation (ID 100010434). She has also received the 2021 Merck’s Award for the Investigation in MS, awarded by Fundación Merck Salud (Spain). In 2015, she received an ECTRIMS Post-doctoral Research Fellowship and has received funding from the UK MS Society. She has also received honoraria from Roche and Novartis, and is a steering committee member of the O’HAND trial and of the Consensus group on Follow-on DMTs. This project has received funding under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 634541 and from the Engineering and Physical Sciences Research Council (EPSRC EP/R006032/1), funding FG. FG is supported by an investigator-initiated study at the Vall d'Hebron Institute of Oncology (Barcelona, Spain), funded by AstraZeneca (PREdICT study). AstraZeneca, Merck, Roche, Novartis and Philips did not influence data acquisition and analysis, result interpretation and the decision to submit this work in its present form to a conference.References
- Le Bihan D. Du mouvement Brownien aux images de la pensée: l'IRM de diffusion [From Brownian motion to mind imaging: diffusion MRI]. Bull Acad Natl Med. 2006 Nov;190(8):1605-27; discussion 1627. French. PMID: 17650747.
- Tofts P. Quantitative MRI of the brain: measuring changes caused by disease, John Wiley & Sons. 2005.
- Lebel C, & Deoni, S. The development of brain white matter microstructure. Neuroimage. 2018;182:207-218.
- Assaf Y, Basser PJ. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. Neuroimage. 2005;27(1):48-58.
- Shaw C, Jensen J, Deardorff R, et al. Comparison of Diffusion Metrics Obtained at 1.5T and 3T in Human Brain With Diffusional Kurtosis Imaging. J Magn Reson Imaging. 2017 Mar;45(3):673-680. doi: 10.1002/jmri.25380. Epub 2016 Jul 12. PMID: 27402163.
- Yoneyama M, Takahara T, Kwee T.C, et al. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse, Magn. Reson. Med. Sci. 2013;12(2):111–119.
- Kasper J.M, Wadhwa V, Scott K.M, et al. SHINKEI-a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging, Eur. Radiol. 2015;25(6):1672–1677.
- Modat M, Cash DM, Daga P, et al. Global image registration using a symmetric block-matching approach. J Med Imaging (Bellingham). 2014;1(2):024003.
- Hansen B, Jespersen SN. Data for evaluation of fast kurtosis strategies, b-value optimization and exploration of diffusion MRI contrast. Sci Data. 2016;3:160072. doi:10.1038/sdata.2016.72.
- Garyfallidis E, Brett M, Amirbekian B, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 2014;8:8.doi: 10.3389/fninf.2014.00008. eCollection 2014.
- Bland J M, Altman D G. Measurement error. BMJ: British medical journal. 1996a;312: 1654.
Figures
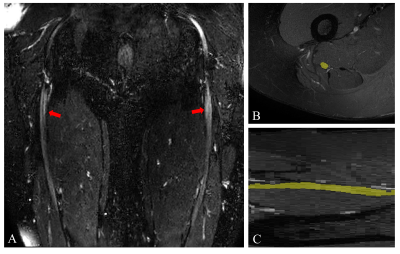
Figure 1. A) Large
field-of-view 3D SHINKEI image showing the sciatic nerves (red arrows) in a
healthy control B) Manual segmentation of the sciatic nerve slice-by-slice in
the axial plane using the fat-suppressed T2-weighted acquisition (binary mask
shown in yellow) C) Segmentation of the sciatic nerve shown in the sagittal
plane (binary mask shown in yellow).
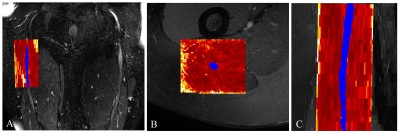
Figure 2. A) Example of the FA map calculated
in the sciatic nerve of a healthy control (right upper thigh) shown in the
coronal plane of the 3D SHINKEI image with the segmented binary mask overlaid
(shown in blue); B) the FA map in the axial plane overlaid on the fat-suppressed
T2-weighted image; C) the FA map in the sagittal plane overlaid on the
fat-suppressed T2-weighted acquisition.
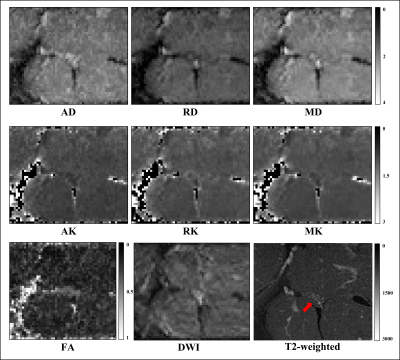
Figure 3. Example of standard
diffusion tensor imaging metrics axial/radial/mean diffusivity (AD/RD/MD), diffusion
kurtosis imaging (DKI) metrics axial/radial/mean kurtosis (AK/RK/MK),
fractional anisotropy (FA), diffusion
weighted image of sciatic nerve and T2-weighted image
showing the sciatic nerve (red arrow) in a
healthy control.
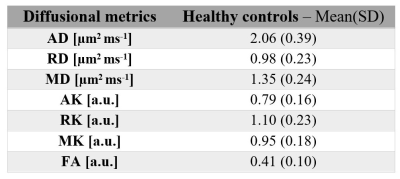
Table 1. Mean
(SD) standard diffusion tensor imaging metrics axial/radial/mean
diffusivity (AD/RD/MD), fractional anisotropy (FA) and DKI metrics axial/radial/mean
kurtosis (AK/RK/MK) in the sciatic nerve of 11 healthy
controls.
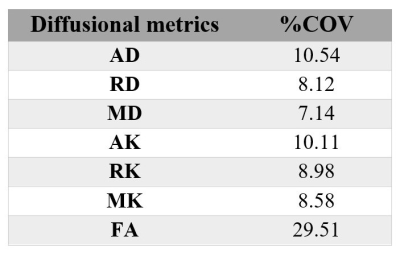
Table
2. The reproducibility
assessment (coefficient of variation [%COV]) in five healthy controls of standard diffusion tensor imaging metrics axial/radial/mean
diffusivity (AD/RD/MD), diffusion kurtosis imaging (DKI) metrics
axial/radial/mean kurtosis (AK/RK/MK) and fractional anisotropy (FA).
DOI: https://doi.org/10.58530/2022/2793