2772
Flow 2.0 - a flexible, scalable, cross-platform post-processing software for real-time phase contrast sequences1CHIMERE UR 7516, Jules Verne University, Amiens, France, 2Medical Image Processing Department, Jules Verne University Hospital, Amiens, France
Synopsis
Real-time phase contrast sequences (RT-PC) has potential value as a scientific and clinical tool in quantifying the effects of respiration on cerebral circulation. To simplify its complicated post-processing process, we developed Flow 2.0 software, which provides a complete post-processing workflow including converting DICOM data, image segmentation, image processing, data extraction, background field correction, anti-aliasing filter, signal processing and analysis and a novel time-domain method for quantifying the effect of respiration on the cerebral circulation. This end-to-end software allows us to quickly, robustly and accurately perform batch process RT-PC and multivariate analysis of the effects of respiration on cerebral circulation.
Introduction
With further development of imaging techniques and instrumentation, real-time phase contrast sequences (RT-PC) have become possible1. Compared to conventional phase-contrast sequences, its most significant feature is the quantification of cerebral blood flow or cerebrospinal fluid (CSF) in "real-time". Although not yet applied in clinical practice, recent studies have shown that RT-PC has a great potential for non-invasive quantification of the effects of respiration on cerebral fluids circulation2-4. However, the fast imaging speed that impairs RT-PC image quality, and a large number of images make it more difficult to post-process RT-PC data. In general, the post-processing of RT-PC data often requires a lot of manual operations and several software to perform the different steps such as region of interest (ROI) segmentation, background field correction, anti-aliasing correction, extraction of flow signals, and finally quantify the effect of respiration on blood flow by comparing respiration and flow signals. To our knowledge, there is no post-processing software specifically dedicated to RT-PC data. Based on the original version5, we have developed Flow 2.0 software integrating all the above-mentioned features and allowing a high level of automation for end-to-end quantification of the effect of respiration on cerebral blood flow.Methods
Flow 2.0 was developed using IDL (Interactive Data Language) and was compiled into a 150Mb executable installation-free program which is available on Windows, Linux and Mac systems. Figure 1 shows the post-processing workflow diagram of the software, it contains two major post-processing steps, the flow quantification (Figure 2) and the quantification of respiratory effects (Figure 3).- Flow quantification
A frequency domain signal processing tool interface that is useful for other processing functions was also developed. An Export function can save all the results in a text file, easily importable by other software.
- Quantification of respiratory effects
Results
Figure 4 shows an example of an in vivo application of the software. The automatic algorithm accurately segmented the CSF Roi in the spinal canal. We can see the flow rate signal and the respiratory quantification results.Discussion & Conclusion
We have developed a post-processing software for RT-PC that integrates the required functions, with a high degree of automation, with low requirements for a priori knowledge of anatomy, high robustness and supporting functional extensions. The software is compatible with the DICOM files generated by the MRI vendors. Our development makes the post-processing of RT-PC substantially easier, while also providing a multivariate approach to analyze the effects of respiration on cerebral fluids circulation.Acknowledgements
This research was supported by equipEX FIGURES (Facing Faces Institute Guilding Research), European Union Interreg REVERT Project, Hanuman ANR-18-CE45-0014 and Region Haut de France.
Thanks to the staff members at the Facing Faces Institute (Amiens, France) for technical assistance.
Thanks to David Chechin from Phillips industry for his scientific support.
References
- Chen L, Beckett A, Verma A, & Feinberg D A. (2015). Dynamics of respiratory and cardiac CSF motion revealed with real-time simultaneous multi-slice EPI velocity phase contrast imaging. Neuroimage, 122, 281-287.
- Aktas G, Kollmeier J M, Joseph A A, Merboldt K D, Ludwig H C, Gärtner J, ... & Dreha-Kulaczewski S (2019). Spinal CSF flow in response to forced thoracic and abdominal respiration. Fluids and Barriers of the CNS, 16(1), 1-8.
- Balédent O, LIU P, Lokossou A, Sidy F, Metanbou S, Makki M (2019). Real-time phase contrast magnetic resonance imaging for assessment of cerebral hemodynamics during breathing. [Oral]. ISMRM 27th Annual Meeting & Exhibition in Montreal, Canada.
- Lloyd R A, Butler J E, Gandevia S C, Ball I K, Toson B, Stoodley M A, & Bilston L E (2020). Respiratory cerebrospinal fluid flow is driven by the thoracic and lumbar spinal pressures. The Journal of Physiology, 598(24), 5789-5805.
- Balédent O, & Idy-peretti I (2001). Cerebrospinal fluid dynamics and relation with blood flow: a magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Investigative radiology, 36(7), 368-377.
- Holland B J, Printz B F, & Lai W W (2010). Baseline correction of phase-contrast images in congenital cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance, 12(1), 1-7.
Figures
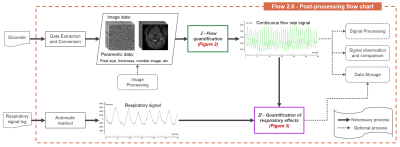
Figure 1: RT-PC post-processing workflow diagram of Flow 2.0. The RT-PC sequence images can be automatically extracted by reading the DICOMDIR file, and the flow rate signal can be extracted by the flow quantification processing phase (green box & figure2). The flow rate signal and the respiratory signal can be used as input parameters to quantify the effects of respiration on the flow signal (purple box & figure 3).
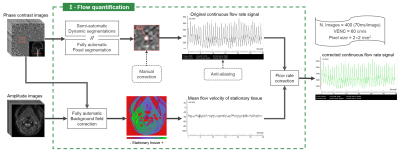
Figure 2: Flow quantification workflow diagram. 400 phase images and 400 amplitude images of the extracranial section were used as input parameters, and the corrected continuous flow rate signal of internal carotid artery (ICA) was used as the output result. Flow 2.0 can extract multiple signals from the ROI, and only the flow rate signal is shown in this figure. The stationary area around the ROI (red contour line) selected by the automatic background field correction algorithm.
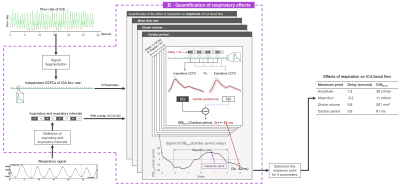
Figure 3: Quantification of respiratory effects workflow diagram. Flow rate signal of ICA and respiratory signal were used as input parameters. A DiffEx-In (parameter, delay) signal is generated for each parameter (amplitude, mean flow, stroke volume, cardiac period) and its maximum value was taken to represent the intensity and delay of the effect of respiration on this parameter.
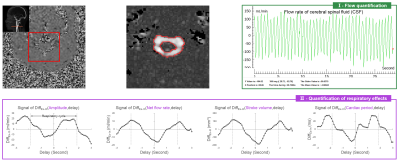
Figure 4: An in vivo application of Flow 2.0 to quantify the effect of respiration on cerebrospinal fluid (CSF) in the extracranial section. VENC = 5cm/s, imaging speed = 96ms/image, Number image = 300. The ROI of CSF can be accurately defined by a fully automated fixed segmentation algorithm, flow rate signal obtained after background field correction (green box). Combining the respiratory signal, the effect of respiration on the four parameters of CSF flow can be obtained (purple box).