2770
Constraint-Based Sequence Optimization in a Scanner-Independent MRI Framework1Fraunhofer Institute for Digital Medicine MEVIS, Bremen, Germany, 2mediri GmbH, Heidelberg, Germany, 3University of Bremen, Bremen, Germany
Synopsis
We demonstrate a constraint-based approach of MRI sequence development in the vendor-independent MRI pulse sequence development framework gammaSTAR and demonstrate this concept in arterial spin labeling by optimizing the timings of the background suppression pulses to minimize the signal contribution of chosen T1 values in the human brain. This concept can raise MRI sequence development to a new abstraction level by, instead of providing exact timings and parameter values, defining physical constraints and conditions to be satisfied by the MRI sequence during an automated sequence generation process.
Introduction
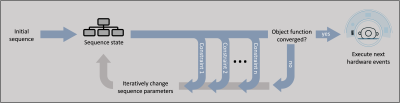
Sequence development for magnetic resonance imaging (MRI) is conventionally performed using complex, platform-specific solutions. During the last years, platform-independent alternatives have attracted attention [1-7].We recently introduced the vendor-agnostic MRI sequence development framework gammaSTAR that allows dynamic sequence changes during the scan without the requirement of pre-defining hardware execution orders [6-8]. In gammaSTAR, MRI sequences are represented as dynamic, hierarchical sequence trees which are interpreted at runtime. The developed graph-based calculations allow sequence parameter definitions to be independent of calculation order. This concept allows to extend the way of implementing MRI sequences to constraint-based approaches in which the user does not define specific timings and parameters, but constraints to be satisfied when automatically generating the MRI sequence. This will eventually lead to a scenario in which, e.g., the parameters of the gradient pulses will not be implemented manually, but composed automatically using the knowledge about the purpose and moments to achieve. In this work, we demonstrate this concept in arterial spin labeling (ASL) by optimizing the timings of the background suppression (BS) pulses to minimize the signal contribution of chosen T1 values in the human brain. Due to the very subtle signal changes to be observed, ASL strongly benefits from a good background suppression, making the optimization of such parameters desirable.
Methods
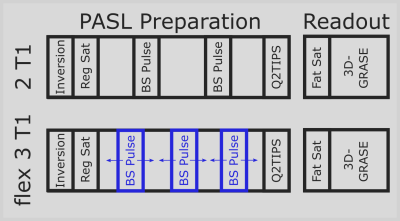
The gammaSTAR framework allows the implementation of constraint-based sequence parameters as alternatives to conventional fixed parameters. During traversal of the sequence tree, these parameters invoke an optimization process inside the related part of the sequence tree. This optimization is based on an object function to be minimized or maximized and the optional definition of constraints that need to be satisfied. For each step of the optimization, all dependent parameters in the sequence tree are updated and, thus, the sequence state is constantly updated during the optimization process. The constraint-based optimization in gammaSTAR uses the COBYLA algorithm of the nlopt library [9-10]. In this work, it is used to implement dynamic background suppression into an ASL sequence [11] for which only a solution to suppress specific combinations of two T1 values can be found analytically (here called 2T1). Suppressing three different T1 values requires numerical minimization of all remaining signal contributions (here called flex3T1).The constraint-based implementation of the flex3T1 method was tested in a gammaSTAR pulsed ASL (PASL) sequence with GRASE readout. After inversion with a FOCI pulse, the acquisition volume is saturated using four saturation pulses with subsequent spoilers. Conventionally, two background suppression FOCI pulses are applied during an inversion time of TI = 1.7 s to suppress signal coming from tissue with T1 = 700 ms and 1400 ms (2T1, Fig. 2 top). A second measurement was performed with three BS pulses instead (flex3T1, Fig. 2 bottom). Besides suppression of the same T1 values of the first measurement, an additional T1 value of 3000 ms is suppressed. Q2TIPS pulses are applied to get a bolus duration of 1500 ms.
Fat saturation was applied before the 3D-GRASE readout with following parameters: TR/TE = 4000/18.3 ms, flip angles = 90° for excitation and 120° for spin echo pulses, ADC readout duration of 0.42 ms, field-of-view = 256×192×128 mm3, matrix size = 64×48×32, EPI factor = 24, partial Fourier factor of 0.75 in slice direction, turbo factor = 12, PAT acceleration factor of 2 in phase direction, which results in 2 segments for one image and a total measurement scan time of about 20 seconds including one prescan and PAT calibration scan. A human brain of a healthy volunteer was measured on a whole-body MRI scanner at 3 Tesla (MAGNETOM Skyra, Siemens Healthineers, Erlangen, Germany) with a 16-channels head coil.
Results
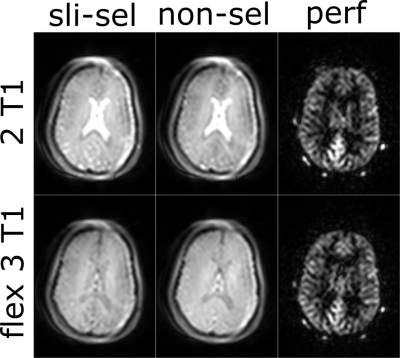
In Figure 3, the in vivo images of the slice-selective and non-selective labeling with the corresponding perfusion-weighted images are shown for the measurement with analytical solution to suppress two T1 values of 700 ms and 1400 ms (top) and the measurement with the flex3T1 background suppression method with an additional suppression of T1 = 3000 ms (bottom). It can be observed that grey and white matter show similar signal values and contrast, whereas cerebrospinal fluid is additionally suppressed in case of the constraint-based flex3T1 method after sequence optimization. The perfusion-weighted image of the latter technique shows higher homogeneity with less CSF fluctuations.Discussion & Conclusion
We demonstrated a way to calculate MRI sequence parameters and timings using a set of constraints to raise MRI sequence development to a new abstraction level. Instead of providing exact timings, parameter values and execution orders, users can define physical constraints to be satisfied by the MRI sequence and the gammaSTAR framework will optimize the measurement accordingly by adapting the sequence during the execution. Because all dependent parameters of the sequence tree are updated with each optimization step, complex dependencies can be considered during this process, eventually making it possible to orchestrate an MRI sequence by only defining the physical properties to be required.Acknowledgements
The authors are grateful to Jörn Huber for valuable discussion and support.References
[1] Jochimsen TH & von Mengershausen M. ODIN-object-oriented development interface for NMR. J Magn Reson 2004;170(1):67-78.
[2] Stöcker T, Vahedipour K, Pfugfelder D, Shah NJ. High-performance computing MRI simulations. Magn Reson Med 2010;64(1):186-93.
[3] Magland JF, Li C, Langham MC, Wehrli FW. Pulse sequence programming in a dynamic visual environment: SequenceTree. Magn Reson Med 2016;75(1):257-65.
[4] Layton KJ, Kroboth S, Jia F, Littin S, Yu H, Leupold J, Nielsen JF, Stöcker T, Zaitsev M. Pulseq: A rapid and hardware‐independent pulse sequence prototyping framework. Magn Reson Med 2017;77(4):1544-52.
[5] Nielsen JF & Noll DC. TOPPE: A framework for rapid prototyping of MR pulse sequences. Magn Reson Med 2018;79(6):3128-34.
[6] Cordes C, Konstandin S, Porter D, Günther M. Portable and platform-independent MR pulse sequence programs. Magn Reson Med 2020;83(4):1277-90.
[7] https://gamma-star.mevis.fraunhofer.de
[8] Hoinkiss DC, Cordes C, Konstandin S & Günther M. Event-Based Traversing of Hierarchical Sequences Allows Real-Time Execution and Arbitrary Looping in a Scanner-Independent MRI Framework. Proc Int Soc Magn Reson Med 2020;1043.
[9] Johnson SG. The NLopt nonlinear-optimization package. http://github.com/stevengj/nlopt
[10] Powell MJD. A direct search optimization method that models the objective and constraint functions by linear interpolation. Advances in Optimization and Numerical Analysis;1994:51-67.
[11] Huber J, Hoinkiss DC & Günther M. Subject-Specific Background Suppression in 3D Pseudo-Continuous Arterial Spin Labeling Perfusion Imaging. Proc Int Soc Magn Reson Med 2020;3283.
Figures