2755
Development of USPIO-DM1-5D3-CF750: An image-guided targeted drug delivery system for prostate cancer therapy1Department of Radiology and Radiological Science, The Johns Hopkins School of Medicine, Baltimore, MD, United States, 2Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, United States, 3Department of Chemical and Biomolecular Engineering, The Johns Hopkins University, Baltimore, MD, United States, 4Laboratory of Structural Biology, Institute of Biotechnology of the Czech Academy of Sciences, Vestec, Czech Republic
Synopsis
Prostate-specific membrane antigen (PSMA) is overexpressed in prostate cancers (PC) compared to normal tissues. Hence, PSMA can be used as a diagnostic biomarker and biological target to deliver drugs in PC therapy. In this study, we have conjugated ultra-small superparamagnetic iron oxide nanoparticles with mertansine (DM1), a chemotherapeutic drug, novel anti-PSMA 5D3 antibody, and NIR CF-750 fluorophore. This multimodality image-guided drug delivery system was evaluated in PC mouse models using in vivo fluorescent imaging and MRI at 9.4T. Promising results encourage further development of targeted image-guided drug delivery platforms for PC therapy.
Purpose
To develop an image-guided and targeted drug delivery system for utilizing ultra-small super paramagnetic iron oxide (USPIO) nanoparticles, 5D3 anti-PSMA monoclonal antibody, mertansine (DM1) anti-tubulin drug, and near-infrared fluorophore for prostate cancer therapy.Methods
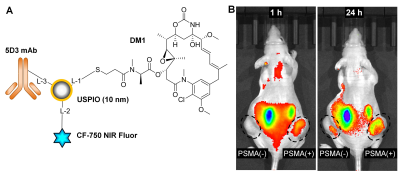
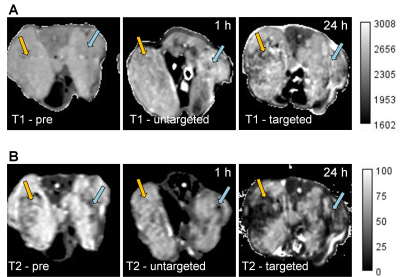
Prostate cancer (PC) is the most common cancer in men. It is associated with the second highest cancer related mortality.1 PC eventually becomes castrate-resistant prostate cancer (CRPC) and progresses rapidly to metastatic form (mCRPC).2 In these mCRPC stages, drugs, such as docetaxel and cabazitaxel lack efficacy and can produce significant toxic effects in healthy tissues and organs. Prostate-specific membrane antigen (PSMA) is overexpressed in practically all malignant PC compared to non-prostatic and non-malignant tissues.3 Its expression is also related to cancer aggressiveness and androgen blockage and deprivation is further enhancing PSMA levels. Hence, PSMA can be used as a biomarker to deliver drugs to PSMA(+) PC. PSMA-specific antibodies, peptides and small molecules are widely used as bioligands to target PSMA for imaging and therapy.4 Novel anti-PSMA monoclonal antibody (mAb), 5D3, has been successfully used for imaging and drug delivery in PSMA(+) PC.5-6 This antibody has higher PSMA binding affinity than other existing PSMA-targeting antibodies.7 Ultra-small superparamagnetic iron oxide (USPIO) nanoparticles are biocompatible, can be detected with high sensitivity by MRI, and have high loading capacity for cargo molecules. Therefore, USPIO platforms are highly relevant for development of MRI-guided drug delivery. Specific delivery of SPIO nanoparticles to target tumors can be achieved by decorating their surface with target-specific mAb.8In this report we have developed a novel USPIO-5D3 targeted MRI-detectable platform to deliver chemotherapeutics to PSMA(+) PC. 5D3 mAb targeted USPIO nanoparticles were also conjugated with mertansine (DM1), a highly-potent anti-tubulin drug, to obtain USPIO-DM1-5D3 drug delivery system. This complex was further conjugated with near-infrared (NIR) fluorophore for optical tracking of the delivery. This final multimodality theranostic image-guided drug delivery system, USPIO-DM1-5D3-CF750 (shown in Figure 1A) combines both therapeutic and image-guided diagnostic capabilities. Biodistribution and tumor uptake of USPIO-DM1-5D3-CF750 were studied in PSMA(±) tumor mouse models. The PSMA(+) and PSMA(-) dual tumor mouse models were prepared by inoculation of PC3-Flu and PC3-PIP cells in the right and left flank of mice, respectively. Mice were injected with USPIO-DM1-5D3-CF750 nano-theranostics (Total-weight dose: 5 mg/kg) i.v. and imaged after 1 h and 24 h using Xenogen in vivo live animal optical imaging system. The results show high tumor uptake of nano-theranostics in PSMA(+) PC3-PIP tumor (Figure 1B). We also observed high liver uptake of the nano-theranostics and renal excretion of CF-750. After 24 h, mice were euthanized and tumors and vital organs, brain, heart, lungs, liver, kidneys, spleen, and intestine were extracted and imaged ex vivo using Xenogen. MRI images were taken after systemic i.v. administration of USPIO-DM1-5D3-CF750 (Total-weight dose: 10 mg/kg) in dual tumor mouse models (Figure 2) using a 9.4T Bruker small animal MRI system. We observed a significant change in T1 contrast in tumors compared to the pre-scan; however, the T1 contrast difference between PSMA(+) and PSMA(-) tumors was not significant (Figure 2A). T2-weighted images showed considerably higher contrast of USPIO-DM1-5D3-CF750 uptake in PSMA(+) tumors compared to PSMA(-) tumor (Figure 2B).
We are currently determining MR relaxation properties of the nano-theranostics and evaluating significance of the tumor uptake. We are also optimizing the conjugation chemistry and evaluating USPIO with different surface functional groups. This study fosters a strong foundation to develop a highly effective image-guided drug delivery system, using biocompatible, high-capacity USPIO nanoparticles, high PSMA affinity 5D3 mAb, and highly potent mertansine (DM1) chemotherapeutics to treat PSMA-overexpressing PC.
Acknowledgements
This study was supported by the DOD (W81XWH-20-1-0429) grant and Emerson Collective Cancer Research Fund (128821-2018).References
1. Siegel, R. L.; Miller, K. D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J Clin. 2020; 70: 7-30.
2. Cho, S.; Zammarchi, F.; Williams, D. G. et al. Antitumor Activity of MEDI3726 (ADCT-401), a Pyrrolobenzodiazepine Antibody-Drug Conjugate Targeting PSMA, in Preclinical Models of Prostate Cancer. Mol Cancer Ther. 2018; 17: 2176-2186.
3. Israeli, R. S.; Powell, C. T.; Corr, J. G. et al. Expression of the Prostate-Specific Membrane Antigen. Cancer Res. 1994; 54: 1807-1811.
4. Boinapally, S.; Ahn, H.; Cheng, B. et al. A Prostate-Specific Membrane Antigen (PSMA)-Targeted Prodrug with a Favorable in vivo Toxicity Profile. Sci. Rep. 2021; 11: Article No. 7114.
5. Hapuarachchige, S. Huang, C. T.; Donnelly, M. C. et al. Cellular Delivery of Bioorthogonal Pretargeting Therapeutics in PSMA-Positive Prostate Cancer. Mol Pharm 2020; 17: 98-108.
6. Colin, T. H. Guo, X.; Barinka, C. et al. Development of 5D3-DM1: A Novel Anti-Prostate-Specific Membrane Antigen Antibody-Drug Conjugate for PSMA-Positive Prostate Cancer Therapy. Mol. Pharmaceutics 2020; 17: 3392–3402.
7. Novakova, Z.; Foss, C. A.; Copeland, B. T. et al. Novel Monoclonal Antibodies Recognizing Human Prostate-Specific Membrane Antigen (PSMA) as Research and Theranostic Tools. Prostate 2017; 77: 749-764.
8. Julien, D. C. Behnke, S.; Wang, G. et al. Utilization of Monoclonal Antibody-Targeted Nanomaterials in the Treatment of Cancer. MAbs. 2011; 3: 467–478.
Figures