2752
Measuring lactate metabolism in vivo in at physiological level using [1-13C]lactate hyperpolarised with an endogenous labile radical precursor1University of Cambridge, Cambridge, United Kingdom, 2General Electric Healthcare, Chalfont St Giles, United Kingdom
Synopsis
A hyperpolarised [1-13C]lactate solution prepared by dynamic nuclear polarization at 7T and 1.4K using photo-irradiated alpha-ketoglutarate as the polarizing agent was injected at low dose in healthy rodents to allow measuring in vivo metabolism in the rodent brain and liver at physiological concentration. As a result of the large 13C polarisation, it was possible to detect metabolic products of [1-13C]lactate in vivo, including [1-13C]pyruvate and 13C-bicarbonate. The observed pyruvate-to-lactate ratios were commensurate with in vitro values previously reported in the literature. The purely endogenous composition of the solution offers the possibility of performing similar measurements in humans.
Introduction
Using hyperpolarised (HP) 13C MR, it is possible to unravel the metabolic processes within cells of healthy and diseased tissue in real time. Traditionally HP 13C MR has been carried out with 13C-pyruvate (typically polarised using persistent trityl radicals). However, it has been suggested that the supraphysiological concentrations that are usually injected can lead to either misinterpretation of the images, due to partial volume effects [1], or directly influence the metabolism under scrutiny through saturation of specific enzymes [2]. We propose to polarise lactic acid using an endogenous radical precursor that achieves polarisation levels such that the HP [1-13C]lactate can be injected at physiological levels, thus allowing the measurement of intracellular metabolism without saturation of any metabolic pathway. To demonstrate this, experiments were carried out in the healthy rodent brain and liver.Methods
Droplets of an 80 % lactic acid and water (w/w) solution doped with 300 mM of alpha-ketoglutaric acid were vitrified by flash freezing in liquid nitrogen prior to being irradiated inside a quartz Dewar with a broadband UV light to create the labile radical on the alpha-KG. Four frozen droplets were loaded into a homebuilt fluid path and sample cup, polarized by dynamic nuclear polarisation inside a 7T cryogen-free polariser, and dissolved and neutralised to pH 7.5 ± 1 prior to injection in the tail vein of anesthetized (2.5% isoflurane) female Wistar rats. MR experiments were carried out in a 9.4 T horizontal bore magnet (Magnex Scientific, Oxford, UK) interfaced to a Bruker console (Bruker, Germany). 13C MR spectra were recorded with a 13C TX/RX surface coil placed over the region of interest using 20-degree flip angle rf pulses separated by a repetition time of 2 s.Results
A radical concentration of 40 mM can be produced in less than 1 min of photoirradiation with ultraviolet-visible light in [1-13C]lactic acid sample containing a starting concentration of only 300 mM of alpha-KG radical precursor. The resulting radical provides a solid-state 13C polarisation of 40 % and the corresponding liquid-state 13C polarisation was measured 30 s post dissolution to be 19 + 2 % (Fig. 1).The real-time evolution of the precursor and its metabolic product could be measured in the liver (Fig.2), yielding a lactate-to-pyruvate ratio of 15 + 2 and a pyruvate-to-bicarbonate ratio of 6 + 2. Experiments performed in the rat brain led to similar ratios: lactate-to-pyruvate was 15 + 2 and pyruvate-to-bicarbonate ratio was 4 + 2.
Discussion
The preliminary results presented show that lactic acid is a promising metabolic marker for assessing cerebral and hepatic metabolism in rodents. The polarisation of the [1-13C ]lactic acid allowed the concentration of the bolus to be reduced sufficiently to produce a plasma concentration of 1.8 + 0.4 mM which is less than a factor of 2 increase on the physiological levels, as basal plasma concentration is on the order of 2 mM for healthy rats [3,4]. Studies in the perfused rat liver cells have led to a lactate-to-pyruvate ratio of 8 – 15 [5], which is similar to the one observed in the present experiments, showing that the injection did not significantly affect the basal concentrations. The main advantage of working at physiological concentration is that neither the transporters not the intracellular enzymes will be saturated by supraphysiological doses, making data interpretation more straightforward. The small bicarbonate detected in the brain raises questions about the effect of isoflurane on cerebral metabolism, an issue that has been previously reported [6,1]. However, the use of the endogenous molecule alpha-KG and its photoinduced labile radical as a polarising agent should allow these experiments to be done in humans and provide the ability to image cerebral bicarbonate as was done with HP [1-13C]pyruvate [7].Conclusion
We show that HP [1-13C]lactate can be polarised to high levels using only endogenous molecules to generate a labile radical. The relatively high natural plasma concentration of lactate (1-2 mM) together with the high levels of polarisation allowed measuring real-time in vivo metabolism at physiological levels following the injection of a low dose (0.12 mmol/kg) of HP [1-13C]lactate. The measured lactate-to-pyruvate ratios in the liver and the brain were found to be close to the expected steady-state value found in the literature.The use of alpha-KG as a labile radical precursor also means that the HP [1-13C]lactate solution used in these experiments could be readily translated for clinical applications.
Acknowledgements
No acknowledgement found.References
1. Miller, J. J., Grist, J. T., Serres, S., Larkin, J. R., Lau, A. Z., Ray, K., ... & Sibson, N. (2018). 13 C pyruvate transport across the blood-brain barrier in preclinical hyperpolarised MRI. Scientific reports, 8(1), 1-15
2. Jin, E. S., Moreno, K. X., Wang, J. X., Fidelino, L., Merritt, M. E., Sherry, A. D., & Malloy, C. R. (2016). Metabolism of hyperpolarized [1‐13C] pyruvate through alternate pathways in rat liver. NMR in Biomedicine, 29(4), 466-474.
3. H. B. Lee, M. D. Blaufox, Journal of Nuclear Medicine 1985, 26, 72-76.
4. J. M. Bastiaansen, H. I. Yoshihara, Y. Takado, R. Gruetter, A. Comment, Metabolomics 2014, 10, 986–994; b) A. P. Chen, J. Y. C. Lau, R. D. A. Alvares, C. H. Cunningham, Magn Reson Med 2015, 73, 2087-2093;
5. H. F. Woods, H. A. Krebs, Biochem J 1971, 125, 129-139.
6. Serrao, E. M., Rodrigues, T. B., Gallagher, F. A., Kettunen, M. I., Kennedy, B. W., Vowler, S. L., ... & Brindle, K. M. (2016). Effects of fasting on serial measurements of hyperpolarized [1‐13C] pyruvate metabolism in tumors. NMR in Biomedicine, 29(8), 1048-1055.
7. Grist, J. T., McLean, M. A., Riemer, F., Schulte, R. F., Deen, S. S., Zaccagna, F., ... & Gallagher, F. A. (2019). Quantifying normal human brain metabolism using hyperpolarized [1–13C] pyruvate and magnetic resonance imaging. Neuroimage, 189, 171-179.
Figures
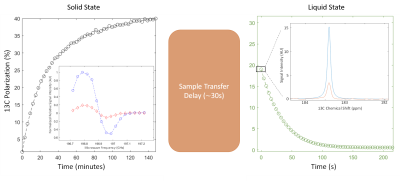
Figure 1. Solid-state and liquid-state polarisation measurement. The sample is dissolved inside the polariser and transferred to the measurement magnet in approximately 30 s. Polarisation build up at the positive maximum microwave frequency of 196.82 GHz with microwave frequency modulation of 2 KHz and 50 MHZ modulation width. Inset is the DNP microwave sweep curve shown with and without microwave frequency modulation (LHS). Liquid state polarisation decay with the first measurement shown inset along with the thermally polarised signal from 1024 transients (RHS).
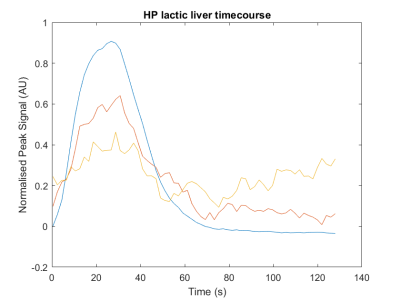
Figure 2. Evolution of the HP [1-13C]lactate precursor and its metabolic products [1-13C]pyruvate and 13C-bicarbonate signals.