2751
Cancer-Induced Metabolic Reprogramming of the Spleen
James D Barnett1, Marie-France Penet1,2, Raj Kumar Sharma1, Balaji Krishnamachary1, Yelena Mironchik1, and Zaver Bhujwalla1,2,3
1Division of Cancer Imaging Research, The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Radiation Oncology and Molecular Radiation Sciences, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
1Division of Cancer Imaging Research, The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Radiation Oncology and Molecular Radiation Sciences, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Our findings demonstrate that tumors drive metabolic alterations in the spleen. These metabolic changes may contribute to immune suppression and poor prognosis.
Introduction
Cancer is a leading cause of death worldwide. Although there have been promising diagnostic and therapeutic advances made in the past decade to reduce cancer mortality, inadequate tumor control remains the sole cause of cancer-related death. Our focus is to investigate the pro-tumorigenic properties of the tumor macroenvironment (TMaE) – the interactions between the tumor and the host as a multi-organ extension of the tumor microenvironment [1]. During tumorigenesis, the spleen – the largest secondary lymphoid organ - becomes a reservoir for hematopoietic and stromal cells that contribute to tumor immune evasion, cancer progression and metastatic cascade [1][2]. Using high resolution 1H magnetic resonance spectroscopy (MRS), we investigated the impact of tumor burden on spleen metabolism in different syngeneic mouse cancer models. Understanding how tumorigenesis affects the metabolic function of vital organs will expand our understanding of what shapes the TMaE and how metabolic intervention can be utilized for anticancer treatments.Methods
Inoculation of 4T1 (mammary carcinoma) and E0771 (mammary carcinoma) were performed orthotopically on female BALB/c and female C57BL/6 mice, respectively. Panc02 (pancreatic adenocarcinoma) cells were inoculated subcutaneously on male C57BL/6 mice. Uninoculated mice of each strain were used as non-tumor-bearing controls (Ctrl). Once tumor volumes reached approximately 400 – 600 mm3, Ctrl and tumor-bearing (TB) mice were euthanized, the spleen and other organs were harvested, freeze clamped and stored at -80 °C for further analyses. Spleens were cryopulverized in liquid nitrogen, and dual phase extractions were conducted using methanol, chloroform and water. 1H MRS analysis of extracted spleen aqueous phases was performed with a Bruker Avance III 750 MHz (17.6T) MR spectrometer to identify water-soluble metabolites. To exclude possible systemic contributions from the blood, plasma 1D NMR metabolomics was performed using the CPMG pulse sequence. Data acquisition was performed using a 5 mm inverse triple-resonance (TXI) probe. Topspin 3.5 software was used for data processing, analysis and quantification.Results
Data summarized in Figure 1 illustrate (A) the average 4T1 (n=4), E0771 (n=5) and Panc02 (n=7) tumor volume at necropsy alongside (B) the corresponding spleen weights of non-tumor-bearing Ctrl female BALB/c (n=5), female C57BL/6 (n=5) and male C57BL/6 (n=10) mice compared to 4T1- (n=4), E0771- (n=5) and Panc02- (n=7) TB mice. Representative spleen 1H MR spectra obtained from 4T1-, E0771-, and Panc02-TB mice with Ctrl for each group are shown respectively in Figures 2, 3 and 4. Figure 5 shows heat maps of percent change between spleen metabolites from (A) female BALB/c Ctrl and 4T1-TB mice, (B) female C57BL/6 Ctrl and E0771-TB mice and (C) male C57BL/6 Ctrl and Panc02-TB mice. Metabolite concentrations were calculated using the internal reference sodium trimethylsilyl propionate (TSP), and all metabolite concentrations were normalized to spleen sample weight. Values represent ± SEM. Two-tailed Student’s t test. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to tumor-bearing mice.Discussion
Splenomegaly was observed in 4T1- and E0771-TB compared to Ctrl, but not in Panc02-TB mice. Distinct, statistically significant changes in metabolites were observed in all TB mouse groups in comparison to Ctrl mice of corresponding strain. These metabolite changes were strikingly different from those identified in the plasma of TB mice compared to Ctrl, ruling out the possibility of any systemic contributions to spleen metabolism. Increases in lactate, alanine, glutamate, glutathione and glycine were common across all groups of TB mice compared to Ctrl. Spleen metabolism determined for different 4T1 tumor volumes revealed that a change in lactate and glutathione metabolism occurred for tumors at 200 - 400 mm3 (data not shown). Further work is being pursued to provide mechanistic context to these findings. Bioenergetics experiments of immune cell populations in the spleen are ongoing to investigate immunosuppression in the splenic microenvironment in parallel with assays to detect aberrant reactive oxygen species production in the spleens of tumor-bearing mice. Our findings highlight the metabolic reprogramming that occurs in the spleen with cancer. These splenic metabolic changes can contribute to immune suppression and poor prognosis. With further investigation, these findings can be used to develop metabolic strategies coupled with frontline treatments to improve cancer patient quality of life.Acknowledgements
Supported by NIH R01CA193365 and R35CA209960.References
1. Steenbrugge J et al., Cancer Research. 2021;81(1)
2. Wu C et al., JCI. 2018;128(8)
Figures
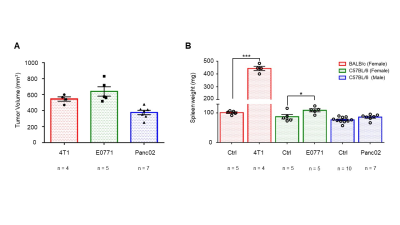
Effects of tumor burden on spleen
weight. (A) Tumor volumes of 4T1 (n=4), E0771 (n=5) and Panc02 (n=7)
tumor-bearing mice upon necropsy. (B) Corresponding spleen weights of
non-tumor-bearing Ctrl female BALB/c (n=5), female C57BL/6 (n=5) and male
C57BL/6 (n=10) mice compared to 4T1 (n=4), E0771 (n=5) and Panc02 (n=7)
tumor-bearing mice. Values represent ± SEM. Two-tailed Student’s t test.
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to tumor-bearing mice.
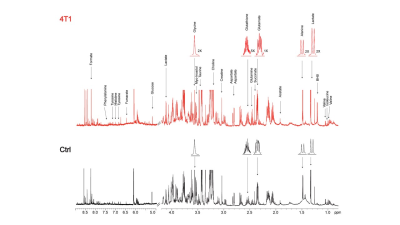
Representative 1H MR spectra of
spleen from 4T1 tumor-bearing and Ctrl (non-tumor-bearing) female BALB/c mice.
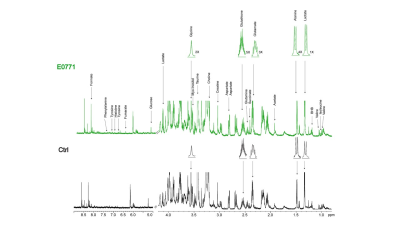
Representative 1H MR spectra of
spleen from E0771 tumor-bearing and Ctrl (non-tumor-bearing) female C57BL/6
mice.
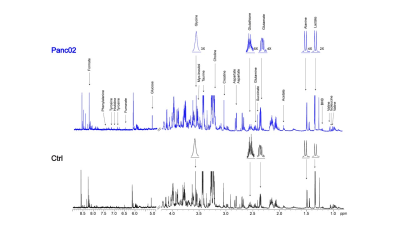
Representative 1H MR spectra of
spleen from Panc02 tumor-bearing and Ctrl (non-tumor-bearing) male C57BL/6 mice.
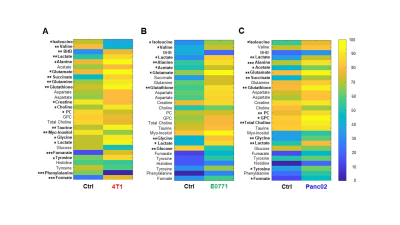
Heat map of percent change between
spleen metabolites from (A) female BALB/c non-tumor bearing Ctrl and 4T1 tumor
bearing mice, (B) female C57BL/6 non-tumor bearing Ctrl and E0771 tumor bearing
mice and (C) male C57BL/6 non-tumor bearing Ctrl and Panc02 tumor bearing mice.
Two-tailed Student’s t test. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to
tumor-bearing mice.
DOI: https://doi.org/10.58530/2022/2751