2750
Preclinical platform for the identification of deuterium magnetic resonance spectroscopy-based biomarkers of tumor metabolism1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Neurological Surgery, University of California San Francisco, San Francisco, CA, United States
Synopsis
Metabolic reprogramming is a fundamental hallmark of cancer, which can be exploited for non-invasive tumor imaging. Deuterium magnetic resonance spectroscopy (2H-MRS) recently emerged as a novel, clinically applicable method of non-invasively monitoring flux from 2H-labeled substrates to metabolic products. However, to date, preclinical studies have been performed in vivo, an endeavor that suffers from low-throughput and potential waste of animal lives, especially in treatment response studies. Here, we demonstrate the ability to quantify metabolism of 2H-MRS probes in live cell suspensions. Our studies will expedite the identification of novel 2H-MRS probes for imaging brain tumors and potentially other cancers.
Introduction
Metabolic reprogramming is a fundamental hallmark of cancer, which can be exploited for non-invasive tumor imaging1,2. Deuterium magnetic resonance spectroscopy (2H-MRS) recently emerged as a novel, non-invasive, translational method of interrogating flux from 2H-labeled substrates to metabolic products3. However, to date, preclinical studies have been performed in vivo3-8, an endeavor which suffers from low-throughput and potential wastage of animal lives, especially when longitudinal studies of treatment response are needed. Developing cell-based assays for monitoring metabolism of 2H-labeled substrates will enhance throughput, lead to the rapid evaluation of new 2H-based probes, and enable identification of treatment response biomarkers, thereby allowing the best 2H-labeled probes to be translated for further in vivo assessment. The goal of this study was to develop a preclinical cell-based platform for quantifying metabolism of 2H-labeled probes in brain tumor models and evaluate the ability of this platform to detect metabolic changes associated with treatment response.Methods
Cell studies: We examined normal human astrocytes (NHACONTROL), patient-derived glioblastoma (GBM1), patient-derived oligodendroglioma (BT88) and patient-derived pediatric diffuse midline glioma (SF7761). All cells were maintained as previously described9-13.Treatment: GBM1 and BT88 cells were treated with a combination of irradiation (10Gy) and temozolomide (TMZ; 100μM) for 72h before the MRS experiment. SF7761 cells were treated for 72h with 2μM ONC20614-16.
2H-MRS of live cells: Cells were incubated in media containing 10mM [U-2H]-pyruvate or 12.5mM [6,6’-2H]-glucose or 5mM [2,3-2H]-fumarate for 72h. Live cells were harvested, suspended in saline in 12mm glass vials and 2H-MR spectra acquired using a 16mm 2H single loop surface coil (DOTY Scientific) on a Varian 14.1T vertical MR scanner (Agilent Technologies). A pulse-acquire sequence (TR=260ms, NA=2500, complex points=512, flip angle=64o, spectral width=2kHz) was used. Corrected amplitudes (for saturation) of fitted water and lactate peaks were converted to concentration in millimolar using the natural abundance HDO signal (12.8mM) collected from a similar vial containing only saline. The latter was determined assuming a 55.5M water concentration and a deuterium natural abundance of 0.0115%3. Data analysis was performed using MestReNova.
Statistical analysis: All results are expressed as mean ± standard deviation. Statistical significance was assessed using an unpaired two-tailed Student’s t-test with p<0.05 considered significant.
Results and Discussion
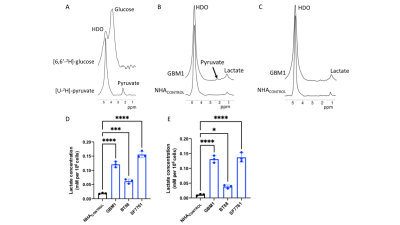
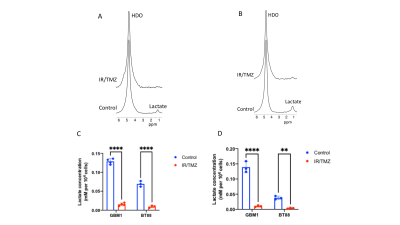
2H-lactate production is higher in glioma cells vs. normal astrocytes Since the Warburg effect, which is characterized by elevated glycolytic flux to lactate, is a metabolic phenotype of cancer17,18, including gliomas, we examined metabolism of [6,6’-2H]-glucose or [U-2H]-pyruvate in patient-derived glioblastoma (GBM1), oligodendroglioma (BT88) or pediatric diffuse midline glioma (SF7761) cells and compared to immortalized normal human astrocytes (NHACONTROL). Following incubation in media containing [U-2H]-pyruvate or [6,6’-2H]-glucose (Fig.1A), 2H-MR spectra obtained from live cell suspensions showed significantly higher 2H-lactate production in GBM1, BT88 and SF7761 cells relative to NHACONTROL (Fig.1B-E).2H-lactate production is reduced in response to therapy Having established our ability to monitor metabolism of 2H-labeled agents in glioma cell models, we examined whether 2H-MRS reports on response to combined radiation and TMZ (TMZ+IR), which is the standard of care for adult glioma patients19,20. We found that 2H-lactate production from [U-2H]-pyruvate or from [6,6’-2H]-glucose was significantly reduced in GBM1 or BT88 cells subjected to irradiation and temozolomide (88.1% drop, p<0.001; N=4 and 86.5% drop, p=0.002; N=3 for GBM1 and BT88 labeled with [U-2H]-pyruvate respectively; 92.6% drop, p=0.006; N=3 and 89.9% drop, p=0.006; N=3 for GBM1 and BT88 labeled with [6,6’-2H]-glucose respectively). These results point to the utility of our cell-based platform for detecting response to chemoradiotherapy (Fig. 2A-D).
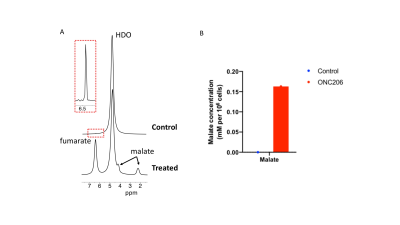
2H-malate production from [2,3-2H]-fumarate report on cell death Previous studies indicate that the imipridone drug ONC206 induces apoptosis in tumors, including diffuse midline gliomas14-16. Studies have also shown that 2H-malate production from [2,3-2H]-fumarate is a sensitive marker of cell death induced by chemotherapy5. We, therefore, examined whether our cell-based assay allows detection of ONC206-mediated cell death in pediatric diffuse midline glioma SF7761 cells. As shown in Fig. 3A-3B, we were able to detect 2H-malate production from [2,3-2H]-fumarate in ONC206-treated cells but not in vehicle controls.
Conclusions
We have, for the first time, developed an assay that allows quantification of the metabolism of 2H-MRS probes in live cell suspensions. Importantly, we have validated the utility of our assay to differentiate glioma cells from normal astrocytes and to assess response to therapy in patient-derived glioma models. Our studies will expedite the identification of novel 2H-MRS probes for imaging brain tumors and potentially other types of cancer.Acknowledgements
This study was supported by NIH R01CA239288, Department of Defense W81XWH201055315 and UCSF Brain Tumor Center Loglio and NICO initiatives.References
[1] Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646-674, doi:10.1016/j.cell.2011.02.013 (2011).
[2] Kim, M. M., Parolia, A., Dunphy, M. P. & Venneti, S. Non-invasive metabolic imaging of brain tumours in the era of precision medicine. Nature Reviews Clinical Oncology 13, 725, doi:10.1038/nrclinonc.2016.108 (2016).
[3] De Feyter, H. M. et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv 4, eaat7314, doi:10.1126/sciadv.aat7314 (2018).
[4] Lu, M., Zhu, X. H., Zhang, Y., Mateescu, G. & Chen, W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37, 3518-3530 (2017).
[5] Hesse, F. et al. Monitoring tumor cell death in murine tumor models using deuterium magnetic resonance spectroscopy and spectroscopic imaging. Proceedings of the National Academy of Sciences of the United States of America 118, doi:10.1073/pnas.2014631118 (2021).
[6] Kreis, F. et al. Measuring Tumor Glycolytic Flux in Vivo by Using Fast Deuterium MRI. Radiology 294, 289-296, doi:10.1148/radiol.2019191242 (2020).
[7] Veltien, A. et al. Simultaneous Recording of the Uptake and Conversion of Glucose and Choline in Tumors by Deuterium Metabolic Imaging. Cancers (Basel) 13, doi:10.3390/cancers13164034 (2021).
[8] von Morze, C. et al. Comparison of hyperpolarized (13) C and non-hyperpolarized deuterium MRI approaches for imaging cerebral glucose metabolism at 4.7 T. Magn Reson Med 85, 1795-1804, doi:10.1002/mrm.28612 (2021).
[9] Kelly, J. J. et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10). Neuro-oncology 12, 745-755, doi:10.1093/neuonc/noq031 (2010).
[10] Mancini, A. et al. Disruption of the beta1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer cell 34, 513-528.e518, doi:10.1016/j.ccell.2018.08.003 (2018).
[11] Sarkaria, J. N. et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clinical cancer research : an official journal of the American Association for Cancer Research 12, 2264-2271, doi:10.1158/1078-0432.Ccr-05-2510 (2006).
[12] Hashizume, R. et al. Characterization of a diffuse intrinsic pontine glioma cell line: implications for future investigations and treatment. J Neurooncol 110, 305-313, doi:10.1007/s11060-012-0973-6 (2012).
[13] Sonoda, Y. et al. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer research 61, 4956-4960 (2001).
[14] Bonner, E. R., Waszak, S. M., Grotzer, M. A., Mueller, S. & Nazarian, J. Mechanisms of imipridones in targeting mitochondrial metabolism in cancer cells. Neuro-oncology 23, 542-556, doi:10.1093/neuonc/noaa283 (2021).
[15] Ishida, C. T. et al. Metabolic Reprogramming by Dual AKT/ERK Inhibition through Imipridones Elicits Unique Vulnerabilities in Glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 5392-5406, doi:10.1158/1078-0432.Ccr-18-1040 (2018).
[16] Wagner, J. et al. Preclinical evaluation of the imipridone family, analogs of clinical stage anti-cancer small molecule ONC201, reveals potent anti-cancer effects of ONC212. Cell Cycle 16, 1790-1799, doi:10.1080/15384101.2017.1325046 (2017).
[17] Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029-1033, doi:10.1126/science.1160809 (2009).
[18] Venneti, S. & Thompson, C. B. Metabolic Reprogramming in Brain Tumors. Annual review of pathology 12, 515-545, doi:10.1146/annurev-pathol-012615-044329 (2017).
[19] Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine 352, 987-996, doi:10.1056/NEJMoa043330 (2005).
[20] Weller, M. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol, doi:10.1038/s41571-020-00447-z (2020).
Figures