2747
ASSESSMENT OF TREATMENT RESPONSE TO DC VACCINE IN RECURRENT GBM PATIENTS USING MULTIPARAMETRIC MRI PREDICTION MODEL1Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
Synopsis
The highly aggressive nature of glioblastoma (GBM) leads to a dismal prognosis, and alternative therapies are being sought. Immunotherapy, such as the Dendritic Cell (DC) vaccine, is currently being evaluated for recurrent GBMs in clinical trials. Follow-up imaging after this immunotherapeutic approach often mimics disease progression on conventional images, making standard MRI evaluation challenging. Our findings indicate that our previously established multiparametric MRI-based prediction model has the capacity to accurately assess response to DC vaccine in recurrent GBM patients, objectively characterizing as either TP or PsP GBM patients, avoiding interruptions in satisfactory treatments, and preventing invasive procedures in PsP cases.
Introduction
Glioblastoma (GBM) is an aggressive tumor, resistant to conventional treatment. Currently, immunotherapy is being used as an alternative treatment modality for recurrent GBMs in clinical trials.1,2,3 These immunotherapeutic approaches, such as dendritic cell (DC) vaccine, harness the patient's immune response to eliminate tumor cells, and in turn, induce profound inflammation at the tumor bed, often referred to as treatment-related changes or pseudoprogression (PsP).4 Standard MR imaging is inadequate for response assessment to immunotherapy in GBM patients even after using refined response assessment criteria secondary to amplified immune response.5,6,7 Thus, there is an urgent need for the development of effective and alternative neuroimaging techniques for accurate response assessment. The purpose of this study was to investigate the potential of our previously established multiparametric physiologic MRI-based predictive model8 in evaluating treatment response in recurrent GBM patients treated with DC vaccine.Methods
Seventeen patients with recurrent GBM (16 IDH wildtype and 1 IDH mutant) treated with DC Vaccine (mean dose= 0.25 mL), who had initially undergone gross total resection of tumor followed by standard chemoradiation therapy, were included in this retrospective IRB-approved study. All patients underwent anatomical imaging, diffusion tensor imaging (DTI), and dynamic susceptibility contrast (DSC)-MR perfusion imaging on a 3T MR system using the parameters described previously.9 In patients in whom tumor specimen was available from repeat surgery/biopsy, malignant features on histopathology were used to identify true progression (TP) (>25% malignant features; n=12) or PsP (<25% malignant features; n=1).10 In the case of non-availability of tissue specimens, >2 consecutive follow-ups standard-of-care MRI scans using updated mRANO criteria11 were used to determine the final diagnosis of TP (n=2) or PsP (n=2). The multiparametric model consisted of a combination of DTI derived fractional anisotropy, linear anisotropy, and DSC-perfusion MRI-derived maximum rCBV from contrast-enhancing regions of tumors in differentiating TP/mixed response from PsP with an accuracy of 90%.8 In this study, we used a combination of these three parameters to compute the progression probabilities (PP) of tumor progression at the time point when tumor progression was suspected on follow-up MRIs, and repeat surgery was being contemplated. The most recent multiparametric MRI preceding re-resection/biopsy was used to calculate PP values which were then correlated with subsequent histopathologic findings. Lesions were considered TP if the predictive PP was ≥ 50% and PsP if predictive PP was ≤ 50%.12 Based upon the PP values, the number of cases correctly classified as TP/PsP were determined. Pearson’s test was performed to find a correlation between our predictive model derived PP values and final diagnosis using histopathology/mRANO criteria. Fisher exact x2 tests were performed to estimate the frequency of MGMT promoter methylation in TP and PsP cases, and Kaplan Meier analyses were used to determine the overall survival using Ki-67%, MGMT methylation, and EGFRvIII gene mutation status as independent variables.Results
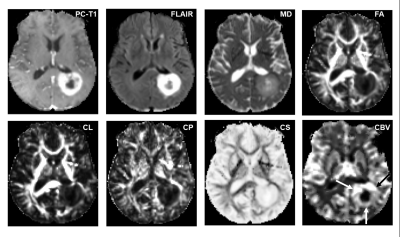
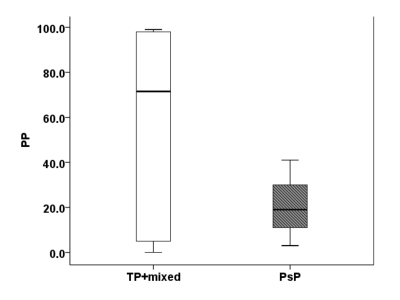
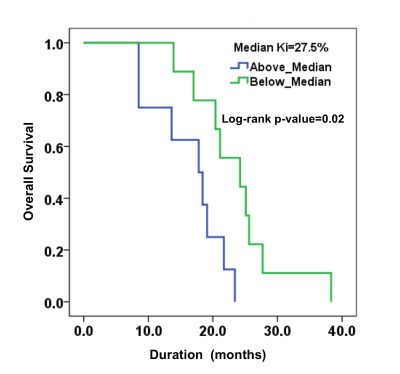
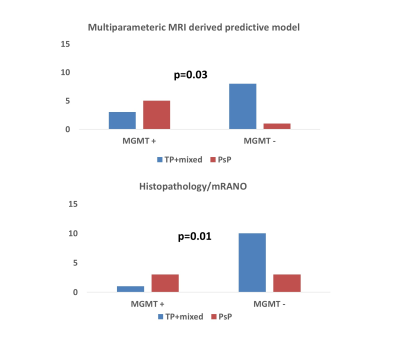
Representative anatomical images, DTI derived parametric maps, and CBV maps are shown in figure 1. While characterizing each lesion as TP or PsP, our predictive model correctly predicted TP in 64% of cases (9/14) and PsP in 100% (3/3), reflecting a significant correlation between PP values and histopathology/mRANO criteria (r = 0.49; p = 0.04), with an overall concordance with the final diagnosis in 70.5% of cases. PP values were higher in TP than in PsP cases (median= 72% vs. 19%, Figure 2). As shown in figure 4, the frequency of patients harboring MGMT promoter methylation was significantly higher in PsP cases when the assessment was performed using PP values derived from multiparametric MRI (P=0.03) or using pathology/mRANO criteria (P=0.01). The overall survival (mean=20.4 months, range=8.5-38.3 months) was significantly higher (P=0.02) in patients with Ki-67 below the median value (median Ki67= 27.5%, range=10-85%, Figure 3). Additionally, trends in longer overall survivals were observed from MGMT methylated and EGFRvIII negative patients compared to their counterparts (P>0.05).Discussion
We report the combined utility of diffusion and perfusion MRI-derived parameters in assessing treatment response to DC vaccine in recurrent GBM patients. A few previous studies have evaluated the potential of these techniques individually in assessing the treatment response to DC vaccine in GBM patients with limited success.13,14,15,16 Therefore, the usage of a single imaging technique or parameter may not always be reliable in evaluating treatment response. It is believed that multiparametric analysis combining the unique strengths of DTI and DSC-MRI techniques could contribute to a more comprehensive assessment of treatment response in these patients. Earlier, we have shown the potential of using multiparametric analysis in evaluating treatment response to EGFRvIII targeted chimeric antigen receptor (CAR) T-cell therapy12 and interleukin-4 receptor-targeted immunotherapy17 in recurrent GBM patients with high accuracy. In the present study, our predictive model correctly classified GBMs as TP or PsP in ~70% of the cases. We believe these findings are promising as GBMs are spatially and temporarily heterogeneous in nature and this heterogeneity increases in the post-treatment settings, further limiting the utility of conventional imaging-based standard response assessment criteria.Conclusion
Our findings indicate that multiparametric MRI may be helpful in assessing response to DC vaccine in recurrent GBM patients. However, this promising finding warrants further validation in the future, with larger clinical trials.Acknowledgements
We thank the patients and their families for their involvement and diligence, the Neuroradiology research core of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA. We also thank Ms. Lisa Desiderio, Ms. Lauren Karpf, and Ms. Eileen Maloney for their technical support and patient recruitment.References
1. Montoya ML, Kasahara N, Okada H. Introduction to immunotherapy for brain tumor patients: challenges and future perspectives. Neuro-Oncology Practice 2020;7:465–76.
2. Desland FA, Hormigo A. The CNS and the Brain Tumor Microenvironment: Implications for Glioblastoma Immunotherapy. Int J Mol Sci 2020;21.
3. Boussiotis VA, Charest A. Immunotherapies for malignant glioma. Oncogene 2018;37:1121–41.
4. Khansur EM, Shah AH, Lacy K, et al. Novel Immunotherapeutics for the Treatment of Glioblastoma: The Last Decade of Research. Cureus 2018;10:e2130.
5. Skolnik AD, Wang S, Gopal PP, et al. Commentary: Pitfalls in the Neuroimaging of Glioblastoma in the Era of Antiangiogenic and Immuno/Targeted Therapy. Front Neurol 2018;9:51.
6. Jackson EF, Barboriak DP, Bidau LM, et al. Magnetic Resonance Assessment of Response to Therapy: Tumor Change Measurement, Truth Data and Error Sources. Translational Oncology 2009;2:211–5.
7. Cruz LCH da, da Cruz LCH, Rodriguez I, et al. Pseudoprogression and Pseudoresponse: Imaging Challenges in the Assessment of Posttreatment Glioma. American Journal of Neuroradiology 2011;32:1978–85.
8. Wang S, Martinez-Lage M, Sakai Y, et al. Differentiating Tumor Progression from Pseudoprogression in Patients with Glioblastomas Using Diffusion Tensor Imaging and Dynamic Susceptibility Contrast MRI. American Journal of Neuroradiology 2016;37:28–36.
9. Chawla S, Wang S, Mohan S, et al. Differentiation of brain infection from necrotic glioblastoma using combined analysis of diffusion and perfusion MRI. J Magn Reson Imaging 2019;49:184–94.
10. Verma G, Chawla S, Mohan S, et al. Three-dimensional echo planar spectroscopic imaging for differentiation of true progression from pseudoprogression in patients with glioblastoma. NMR Biomed 2019;32:e4042.
11. Ellingson BM, Wen PY, Cloughesy TF. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017;14:307–20.
12. Wang S, O’Rourke DM, Chawla S, et al. Multiparametric magnetic resonance imaging in the assessment of anti-EGFRvIII chimeric antigen receptor T cell therapy in patients with recurrent glioblastoma. British Journal of Cancer 2019;120:54–6.
13. Vrabec M, Van Cauter S, Himmelreich U, et al. MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology 2011;53:721–31.
14. Stenberg L, Englund E, Wirestam R, et al. Dynamic susceptibility contrast-enhanced perfusion magnetic resonance (MR) imaging combined with contrast-enhanced MR imaging in the follow-up of immunogene-treated glioblastoma multiforme. Acta Radiologica 2006;47:852–61.
15. Ceschin R, Kurland BF, Abberbock SR, et al. Parametric Response Mapping of Apparent Diffusion Coefficient as an Imaging Biomarker to Distinguish Pseudoprogression from True Tumor Progression in Peptide-Based Vaccine Therapy for Pediatric Diffuse Intrinsic Pontine Glioma. American Journal of Neuroradiology 2015;36:2170–6.
16. Antonios JP, Soto H, Everson RG, et al. Detection of immune responses after immunotherapy in glioblastoma using PET and MRI. Proceedings of the National Academy of Sciences 2017;114:10220–5.
17. Mohan S, Wang S, Chawla S, et al. Multiparametric MRI assessment of response to convection-enhanced intratumoral delivery of MDNA55, an interleukin-4 receptor targeted immunotherapy, for recurrent glioblastoma. Surg Neurol Int 2021;12:337.
Figures