2738
Heroin addiction and relapse-induced axonal transport impairment in the brain and detected by in vivo MRI1Department of Radiology., The Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming, China, 2Department of Radiology, The Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming, China, 3Philips Healthcare China, Guangzhou, China
Synopsis
Heroin plays role in heroin-induced cognitive dysfunction is unclear. We used manganese-enhanced magnetic resonance imaging for to evaluate the effect of heroin on axon transport in the body.
Purpose
Drug addiction has become a considerable health problem due to the abuse drug and toxicity of the drug being abused and the resultant neuronal death and axon degeneration. Though it is a highly addictive drug, the effects of heroin on axonal transport function remain unclear. The aim of this study was to use magnetic resonance imaging (MRI) to characterize heroin addiction (HA)- and heroin-relapse (HR)-induced axon transport impairment and to explore the underlying mechanisms.Methods
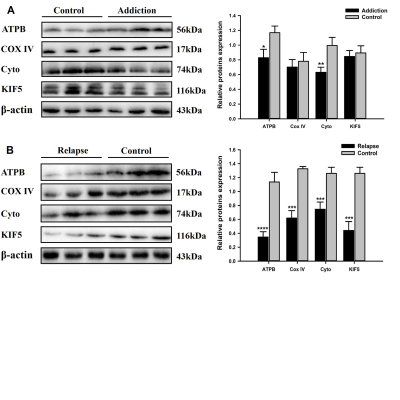
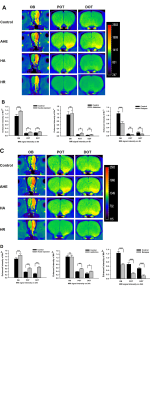
Heroin injections were administered to rats at different times and doses to establish HA and HR rodent models. Heroin-induced learning and memory dysfunction were evaluated by observing the rats in the Morris water maze. MRI was used to dynamically evaluate axonal transport from the olfactory pathway. The protein levels of ATPB, KIF5, COX IV and cytoplasmic dynein were assessed by western blot. TEM was used to observe ultrastructure changes. Neurofilament heavy chain (NF-H) protein levels were analyzed by immunofluorescence staining.Result
HA model rats, and especially HR model rats, showed worse spatial learning and memory abilities than control rats. The escape latency of HR model rats was significantly increased, and the number of crossings and the stay time in the quadrant decreased significantly compared with that of HA model rats and control rats. The transport rate of Mn2+ in HA model rats was accelerated. HR model rats had a severely insufficient capacity for Mn2+ uptake and transport, and their axonal transport rate was significantly reduced compared to that of control rats (P<0.001). The levels of cytoplasmic dynein and KIF5 in rats in the HR group were significantly decreased (P<0.001), and the levels of energy-related proteins, including COX IV and ATPB, were lower than those in rats in the control group (P<0.001). The ultrastructure of the brains of heroin-exposed rats became abnormal, with neuronal apoptosis and mitochondrial disorders. Heroin-induced decreased expression of NF-Hs, and the staining intensity on the tissues from HA and HR model rats was significantly reduced (P<0.05).Conclusion
Long-term relapse of heroin use can cause severe axonal transport disorders, which can be detected by MRI. The reduction in motor proteins and mitochondrial disorders may be the main reasons for this axonal transport damage. Thus, MRI is a potential tool for visualizing axon transport in individuals experiencing drug addiction, which also provides a new direction for the evaluation of addictive drug withdrawal.Acknowledgements
No acknowledgement found.References
[1]. Büttner A, Mall G, Penning R, et al. The neuropathology of heroin abuse. [J].Forensic Sci Int. 2000; 113(1-3):435-442.
[2]. Kumar N, Bhalla MC, Frey JA, et al. Intraparenchymal hemorrhage after heroin use. [J].Am J Emerg Med. 2015; 33(8):1109.e1103-1104.
[3]. Gardini S, Venneri A. Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. [J].Brain Res Bull. 2012; 87(2-3):205-211.
[4]. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. [J].Acta Neuropathol. 2014; 127(1):91-107.
[5]. Ma X, Qiu Y, Tian J, et al. Aberrant default-mode functional and structural connectivity in heroin-dependent individuals. [J].PLoS One. 2015; 10(4):e0120861.
[6]. Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate use. [J].Neuropsychol Rev. 2007; 17(3):299-315.
[7]. Ornstein TJ, Iddon JL, Baldacchino AM, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. [J].Neuropsychopharmacology. 2000; 23(2):113-126.
[8]. Kousik SM, Carvey PM, Napier TC. Methamphetamine self-administration results in persistent dopaminergic pathology: implications for Parkinson's disease risk and reward-seeking. [J].Eur J Neurosci. 2014; 40(4):2707-2714.
[9]. Fitzpatrick RE, Rubenis AJ, Lubman DI, et al. Cognitive deficits in methamphetamine addiction: Independent contributions of dependence and intelligence. [J].Drug Alcohol Depend. 2020; 209(107891. [10]. Potvin S, Pelletier J, Grot S, et al. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. [J].Addict Behav. 2018; 80(154-160.
[11]. Bassiony MM, Youssef UM, Hassan MS, et al. Cognitive Impairment and Tramadol Dependence. [J].J Clin Psychopharmacol. 2017; 37(1):61-66.
[12]. Guo W, Stoklund Dittlau K, Van Den Bosch L. Axonal transport defects and neurodegeneration: Molecular mechanisms and therapeutic implications. [J].Semin Cell Dev Biol. 2020; 99(133-150.
[13]. Mandal A, Drerup CM. Axonal Transport and Mitochondrial Function in Neurons. [J].Front Cell Neurosci. 2019; 13(373.
[14]. Sau D, Rusmini P, Crippa V, et al. Dysregulation of axonal transport and motorneuron diseases. [J].Biol Cell. 2011; 103(2):87-107.
[15]. Martínez-Mármol R, Mohannak N, Qian L, et al. p110δ PI3-Kinase Inhibition Perturbs APP and TNFα Trafficking, Reduces Plaque Burden, Dampens Neuroinflammation, and Prevents Cognitive Decline in an Alzheimer's Disease Mouse Model. [J].J Neurosci. 2019; 39(40):7976-7991.
[16]. Barnett MH, Miller LA, Reddel SW, et al. Reversible delayed leukoencephalopathy following intravenous heroin overdose. [J].J Clin Neurosci. 2001; 8(2):165-167.
[17]. Sempere AP, Posada I, Ramo C, et al. Spongiform leucoencephalopathy after inhaling heroin. [J].Lancet. 1991; 338(8762):320.
[18]. Bora E, Yücel M, Fornito A, et al. White matter microstructure in opiate addiction. [J].Addict Biol. 2012; 17(1):141-148.
[19]. Büttner A, Rohrmoser K, Mall G, et al. Widespread axonal damage in the brain of drug abusers as evidenced by accumulation of beta-amyloid precursor protein (beta-APP): an immunohistochemical investigation. [J].Addiction. 2006; 101(9):1339-1346.
[20]. Anthony IC, Norrby KE, Dingwall T, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. [J].Brain. 2010; 133(Pt 12):3685-3698.
[21]. Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. [J].Acta Neuropathol. 2017; 133(5):665-704.
[22]. Almeida-Corrêa S, Czisch M, Wotjak CT. In Vivo Visualization of Active Polysynaptic Circuits With Longitudinal Manganese-Enhanced MRI (MEMRI). [J].Front Neural Circuits. 2018; 12(42.
[23]. Uselman TW, Barto DR, Jacobs RE, et al. Evolution of brain-wide activity in the awake behaving mouse after acute fear by longitudinal manganese-enhanced MRI. [J].Neuroimage. 2020; 222(116975.
[24]. Lin YJ, Koretsky AP. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. [J].Magn Reson Med. 1997; 38(3):378-388.
[25]. Silva AC, Bock NA. Manganese-enhanced MRI: an exceptional tool in translational neuroimaging. [J].Schizophr Bull. 2008; 34(4):595-604.
[26]. Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. [J].Magn Reson Med. 1998; 40(5):740-748.
[27]. Deng W, Faiq MA, Liu C, et al. Applications of Manganese-Enhanced Magnetic Resonance Imaging in Ophthalmology and Visual Neuroscience. [J].Front Neural Circuits. 2019; 13(35.
[28]. Li Y, Xia B, Li R, et al. Expression of brain-derived neurotrophic factors, neurotrophin-3, and neurotrophin-4 in the nucleus accumbens during heroin dependency and withdrawal. [J].Neuroreport. 2017; 28(11):654-660.
[29]. Li Y, Xia B, Li R, et al. Changes in Expression of Dopamine, Its Receptor, and Transporter in Nucleus Accumbens of Heroin-Addicted Rats with Brain-Derived Neurotrophic Factor (BDNF) Overexpression. [J].Med Sci Monit. 2017; 23(2805-2815.
[30]. Pu H, Wang X, Zhang J, et al. Cerebellar neuronal apoptosis in heroin-addicted rats and its molecular mechanism. [J].Int J Clin Exp Pathol. 2015; 8(7):8260-8267.
[31]. Kreek MJ, Levran O, Reed B, et al. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. [J].J Clin Invest. 2012; 122(10):3387-3393.
[32]. Klein G, Juni A, Arout CA, et al. Acute and chronic heroin dependence in mice: contribution of opioid and excitatory amino acid receptors. [J].Eur J Pharmacol. 2008; 586(1-3):179-188.
[33]. Leppert W. Emerging therapies for patients with symptoms of opioid-induced bowel dysfunction. [J].Drug Des Devel Ther. 2015; 9(2215-2231.
[34]. Morie KP, Nich C, Hunkele K, et al. Alexithymia level and response to computer-based training in cognitive behavioral therapy among cocaine-dependent methadone maintained individuals. [J].Drug Alcohol Depend. 2015; 152(157-163.
[35]. Kitanaka J, Kitanaka N, Hall FS, et al. Memory impairment and reduced exploratory behavior in mice after administration of systemic morphine. [J].J Exp Neurosci. 2015; 9(27-35.
[36]. Marks WD, Paris JJ, Barbour AJ, et al. HIV-1 Tat and Morphine Differentially Disrupt Pyramidal Cell Structure and Function and Spatial Learning in Hippocampal Area CA1: Continuous versus Interrupted Morphine Exposure. [J].eNeuro. 2021; 8(3).
[37]. Mitrović SM, Dickov A, Vučković N, et al. The effect of heroin on verbal memory. [J].Psychiatr Danub. 2011; 23(1):53-59.
[38]. Bao G, Kang L, Li H, et al. Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. [J].Neuropsychopharmacology. 2007; 32(8):1738-1749.
[39]. Aimino MA, Coker CR, Silberman Y. Acute ethanol modulation of neurocircuit function in the nucleus of the tractus solitarius. [J].Brain Res Bull. 2018; 138(5-11.
[40]. Minoshima S, Cross D. In vivo imaging of axonal transport using MRI: aging and Alzheimer's disease. [J].Eur J Nucl Med Mol Imaging. 2008; 35 Suppl 1(S89-92.
[41]. Naughton SX, Hernandez CM, Beck WD, et al. Repeated exposures to diisopropylfluorophosphate result in structural disruptions of myelinated axons and persistent impairments of axonal transport in the brains of rats. [J].Toxicology. 2018; 406-407(92-103.
[42]. Sharma R, Buras E, Terashima T, et al. Hyperglycemia induces oxidative stress and impairs axonal transport rates in mice. [J].PLoS One. 2010; 5(10):e13463.
[43]. Gibbs KL, Greensmith L, Schiavo G. Regulation of Axonal Transport by Protein Kinases. [J].Trends Biochem Sci. 2015; 40(10):597-610.
[44]. Morfini G, Schmidt N, Weissmann C, et al. Conventional kinesin: Biochemical heterogeneity and functional implications in health and disease. [J].Brain Res Bull. 2016; 126(Pt 3):347-353.
[45]. Karle KN, Möckel D, Reid E, et al. Axonal transport deficit in a KIF5A( -/- ) mouse model. [J].Neurogenetics. 2012; 13(2):169-179.
[46]. Maday S, Twelvetrees AE, Moughamian AJ, et al. Axonal transport: cargo-specific mechanisms of motility and regulation. [J].Neuron. 2014; 84(2):292-309.
[47]. Middlemore-Risher ML, Adam BL, Lambert NA, et al. Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons. [J].J Pharmacol Exp Ther. 2011; 339(2):341-349.
[48]. Akbari M, Kirkwood TBL, Bohr VA. Mitochondria in the signaling pathways that control longevity and health span. [J].Ageing Res Rev. 2019; 54(100940.
[49]. Zinsmaier KE, Babic M, Russo GJ. Mitochondrial transport dynamics in axons and dendrites. [J].Results Probl Cell Differ. 2009; 48(107-139.
[50]. Morelli AM, Ravera S, Panfoli I. The aerobic mitochondrial ATP synthesis from a comprehensive point of view. [J].Open Biol. 2020; 10(10):200224.
[51]. Fernandez A, Meechan DW, Karpinski BA, et al. Mitochondrial Dysfunction Leads to Cortical Under-Connectivity and Cognitive Impairment. [J].Neuron. 2019; 102(6):1127-1142.e1123.
[52]. Lee Y, Lee BH, Yip W, et al. Neurofilament Proteins as Prognostic Biomarkers in Neurological Disorders. [J].Curr Pharm Des. 2020; 25(43):4560-4569.
Figures
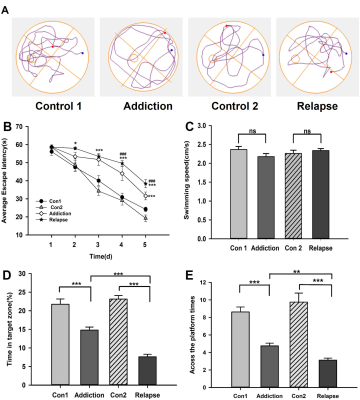
The effect of heroin on the learning and memory abilities of rats in the MWM test.
The navigation test showed the escape latency of rats after heroin exposure was prolonged compared to control (B). The speed among all groups did not show difference (C). In the probe test, the swimming trajectory after evacuating the platform (A), the time spent in the target zone (D) and the number of crossings (E). Significant expression differences are represented as *P < 0.05, **P < 0.01, ***P < 0.001, in escape latency; # indicates the comparison between HR and HA rats; # # #P<0.001.