2736
Brain morphometry abnormalities in children with Childhood Apraxia of Speech.1IRCCS Stella Maris Foundation, Pisa, Italy
Synopsis
Childhood Apraxia of Speech (CAS) is a pediatric speech sound disorder in which precision and consistency of speech movements are impaired in absence of neuromuscular and structural deficit. The cause of CAS remains poorly understood and few neuroimaging studies still exist in children with CAS. In this case-control study we compared both at ROI and voxel level the cerebral gray matter of CAS and healthy controls subjects. The statistical analyses show that gray matter in CAS children is increased in areas of motor circuitries subserving speech and in areas involving sensorimotor learning.
INTRODUCTION
Childhood Apraxia of Speech (CAS) is a pediatric speech sound disorder in which precision and consistency of speech movements are impaired in absence of neuromuscular and structural deficit (ASHA2007). The core deficit involves planning and/or programming the spatio-temporal parameters of movement sequences (ASHA 2007; Maasenn 2010; Shriberg 2010) The cause of CAS remains poorly understood and few neuroimaging studies still exist in children with CAS. Most children with idiopathic CAS show no frank brain structural alterations on clinical MRI. However, there are some recent data documenting the presence of microstructural abnormalities in small samples of children with CAS (Kadis 2014, Fiori 2016, Pigdon 2019, Conti 2020), even if no repeatable results are available yet, given the small samples and the heterogeneity of patients.METHODS
A group of 72 children with CAS, [mean age ± SD = 6.1 ± 2.1 years; age range = 2.8–11.2 years] and a group of 30 healthy control (HC) children matched by age, gender [mean age ± SD = 6.5 ± 2.6 years; age range = 2.6–12.7 years] were chosen for this case–control study. Children with CAS underwent a comprehensive speech and language assessment. The CAS diagnosis was reached by a multidisciplinary team in accordance with the three ASHA criteria and in presence of at least 4 out of 10 Strand’s features (Murray et al 2015) MRI data were acquired using a GE 1.5 T Signa System (GE Healthcare) at IRCCS Stella Maris Foundation Within the MRI protocol for children, a whole-brain fast spoiled gradient recalled acquisition in the steady-state T1- weighted series (FSPGR) was collected in the axial plane, yielding to contiguous axial slices with voxel size of 1 × 1 × 1 mm. FreeSurfer 6.0 recon-all pipeline was used for the segmentation of cortical and sub-cortical brain structures according to the Desikan-Killiany atlas parcellation from T1-weighted MR images. The statistical examination of deep structures volumes, cortical volumes and cortical thicknesses was performed using a multi-way analysis of variance (ANOVA) test. A comparison between CAS and control subjects was performed at ROI level on the entire data set using sex, age and intracranial volume as covariate. A p-value of 0.05 (with and without FDR correction) was considered to reject the null hypothesis of equal means between the groups. Moreover, a Voxel Based Morphometry (VBM) analysis, using the DARTEL algorithm (Ashburner 2000, 2007), was carried on, in order to compare the local concentration of gray matter (GM) between CAS and control subjects.RESULTS
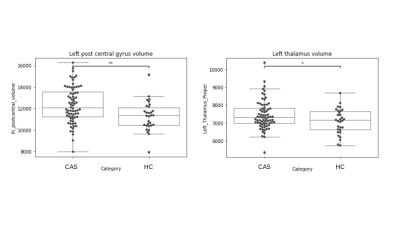
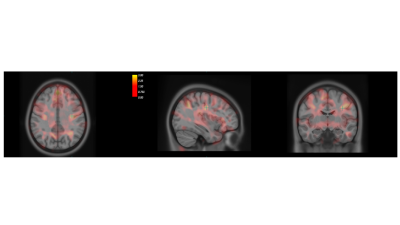
Both ROI-based and voxel-wise analyses revealed structural alterations in CAS population, with an increase of gray matter with respect to age-matched controls. Figure 1 shows the distributions of the Left post central gyrus volumes and Left Thalamus volumes for CAS and HC subjects.ANOVA analysis without FDR correction showed significantly greater GM volumes in CAS subjects with respect to controls in several ROIs such as: the paracentral lobule (left and right) , the postcentral cortex (left and right), the precentral cortex (left and right), the pars opercularis (left and right), the precuneus (left and right), the superior parietal cortex (left and right), the superior frontal cortex (left and right), the left temporal pole, the left supramarginal cortex, the left insula, the left posterior cingulate, the right caudal middle frontal gyrus, the right isthmus of the cingulate and the right lateral orbital cortex. The alterations at cortical thickness level were identified in the postcentral cortex, in the precuneus, in the rostral middle frontal and in the superior parietal cortex. Among the subcortical structures, altered volumes were detected in left and right thalami. FDR correction limited the statistically significant findings to the left postcentral volume and to the right and left thalami volumes only. In accordance to the ROI based analysis, the VBM comparison showed an increased gray matter concentration in CAS subjects bilaterally in the precentral and postcentral gyri, in paracentral lobules, in the superior parietal cortex and in the posterior cingulate, Figure 2 shows the map of T statistics at voxel levels of the CAS vs HC comparison.
DISCUSSION
Our results provide evidence in support of the presence of morphometric brain abnormalities in children with CAS compared to controls. These alterations involved both cortical and subcortical structures bilaterally. We found alterations in motor circuitries subserving speech and in areas involving sensorimotor circuitries. Considering the regions that remain significantly altered after FDR corrections, an area of particular interest is the postcentral gyrus which includes primary somatosensory cortex, a brain region responsible for proprioception including face, lips, and tongue (Kropf 2019). At subcortical level thalamus together with others subcortical structures has been described to play a crucial role in the coordination of sensorimotor behavior such as fluent speech production (Neef 2018). The participation of the thalamus as an integrant element in the language processing circuit has general acceptance. Basal ganglia are involved in motor processing, including articulation, and that the thalamus plays a role in those language functions which involve verbal memory, even if the very specific way in which such structures participate in language remains controversial (Radanovic 2003). Overall, these findings support the hypothesis that different neural systems may be involved in the specific deficits related to CAS.Acknowledgements
No acknowledgement found.References
American Speech-Language-Hearing Association. (2007). Childhood apraxia of speech [Technical Report]. Available from www.asha.org/policy/
Maassen, B., Nijland, L., & Terband, H. (2010). Developmental models of childhood apraxia of speech. In B. Maassen, & P. van Lieshout (Eds.), Speech Motor Control. New developments in basic and applied research (pp. 243 - 258). Oxford University Press.
Shriberg LD, Fourakis M, Hall SD, et al. Extensions to the Speech Disorders Classification System (SDCS). Clin Linguist Phon. 2010;24(10):795-824.
Kadis DS, Goshulak D, Namasivayam A, et al. Cortical thickness in children receiving intensive therapy for idiopathic apraxia of speech. Brain Topogr. 2014;27(2):240-247.
Fiori S, Guzzetta A, Mitra J, Pannek K, Pasquariello R, Cipriani P, Tosetti M, Cioni G, Rose SE, Chilosi A. Neuroanatomical correlates of childhood apraxia of speech: A connectomic approach. Neuroimage Clin. 2016 Nov 4;12:894-901.
Pigdon L, Willmott C, Reilly S, Conti-Ramsden G, Gaser C, Connelly A, Morgan AT. Grey matter volume in developmental speech and language disorder. Brain Struct Funct. 2019 Dec;224(9):3387-3398.
Conti E, Retico A, Palumbo L, et al. Autism Spectrum Disorder and Childhood Apraxia of Speech: Early Language-Related Hallmarks across Structural MRI Study. J Pers Med. 2020;10(4):275. Published 2020 Dec 12.
Murray E, McCabe P, Heard R, Ballard KJ. Differential diagnosis of children with suspected childhood apraxia of speech. J Speech Lang Hear Res. 2015 Feb;58(1):43-60.
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007 Oct 15;38(1):95-113.
Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000 Jun;11(6 Pt 1):805-21.
Kropf E, Syan SK, Minuzzi L, Frey BN. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz J Psychiatry. 2019 May-Jun;41(3):261-269.
Neef NE, Bütfering C, Auer T, Metzger FL, Euler HA, Frahm J, Paulus W, Sommer M. Altered morphology of the nucleus accumbens in persistent developmental stuttering. J Fluency Disord. 2018 Mar;55:84-93.
Radanovic M, Scaff M. Speech and language disturbances due to subcortical lesions. Brain Lang. 2003 Mar;84(3):337-52.