2735
Sex-specific effects of prenatal tobacco exposure on cognitive and brain morphometry measures in preadolescent children1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Department of Otorhinolaryngology, University of Maryland School of Medicine, Baltimore, MD, United States, 3Department of Pediatrics, University of Maryland School of Medicine, Baltimore, MD, United States, 4Department of Neurology, University of Maryland School of Medicine, Baltimore, MD, United States, 5Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Prenatal tobacco exposure (PTE) can affect the offspring cognitive and brain development outcomes. Our study evaluated children who had PTE in the Adolescent Brain and Cognitive Development (ABCD) study and found poorer overall cognitive scores, especially working memory scores, lower cortical surface areas and subcortical volumes, with smaller surface areas in the posterior cingulate (PCC) and entorhinal cortices, the lingual and inferior parietal gyri, and abnormally smaller volumes in the thalamus and nucleus accumbens. Furthermore, we found that only PTE-girls had significantly smaller surface areas in the postcentral gyrus, while only PTE-boys had smaller volumes in the posterior corpus callosum.
Introduction
Prenatal tobacco exposure (PTE), with a global prevalence of 1.7%1, is associated with poorer cognitive function, especially attention, and sex-specific effects on brain metabolites, morphometry, microstructure, and cognitive development2. However, the brain regions and extent of sex-specific effects associated with PTE in the brain remain unclear. Therefore, we compared brain and neurocognitive measures between boys and girls with and without PTE, using the ABCD study baseline data set.Methods
We used the baseline dataset from 11,609 children-parent dyads participating in the ongoing longitudinal ABCD study, at 21 sites in the US. , including comprehensive questionnaires assessing medical history, substance use, mental health, and sociodemographic information, along with multiparametric MRI and cognitive measures3. High-resolution structural MRIs (Siemens: T1-weighted MP-RAGE, TR/TE/TI=2500/2.88/1060 ms, 1-mm isotropic resolution, FOV 256 x256, matrix 256x256 and 176 slices; and equivalent parameters from Philips and General Electric scanners) were acquired with 3 T scanners at 21 sites. Images were processed using FreeSurfer v5.3 and the Desikan-Killiany atlas to measure cortical thickness and surface areas in 27 cortical regions, and volumes in 10 subcortical regions.Uncorrected t-scores from the tests in the NIH Toolbox® were used to evaluate the seven cognitive domains (vocabulary, attention, working memory, executive function, episodic memory, processing speed, reading skills), which generated composite scores for crystallized intelligence, fluid intelligence, and overall cognition. We used linear mixed models and emmeans in R (version 4.1.1) to: (1) evaluate whether children with PTE had altered brain and cognitive measures; 2) examine sex-specific PTE effects on cognition and morphometry. All analysis covaried for the child’s age and race, parental education, family income, prenatal alcohol and marijuana exposure, hemisphere (for brain measures), intercranial volume (for subcortical volumes), and study site and scanner manufacturer. We used false-discovery rate to correct for multiple comparisons, with significance at corrected-p< 0.05.
Results
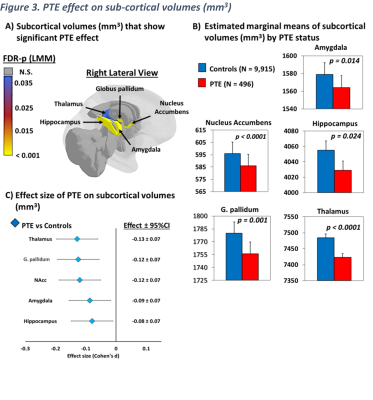
At baseline, youth were 109.3±7.6 months of age. More than half of youth were boys in both groups (controls: 52.3%; PTE: 51.6%) and more PTE children were Black/African Americans (p<0.001). When compared to controls, more parents of PTE-children reported annual incomes below $50K and lower education levels (p<0.001). More PTE children were exposed to other substances (Table 1).Cognition: Compared to unexposed children, PTE-children had poorer performance on executive function (p=0.018), working memory (p=0.003), episodic memory (p=0.015), reading skills (p=0.015), crystallized intelligence (p=0.019), fluid intelligence (p = 0.014) and overall cognition (p=0.003), Figure 1A. PTE effect sizes (Cohen’s d) remained significant after adjusting for covariates (Figure 1B).
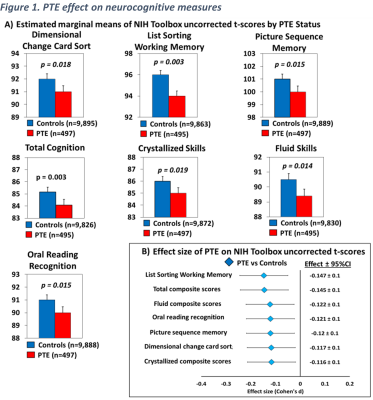
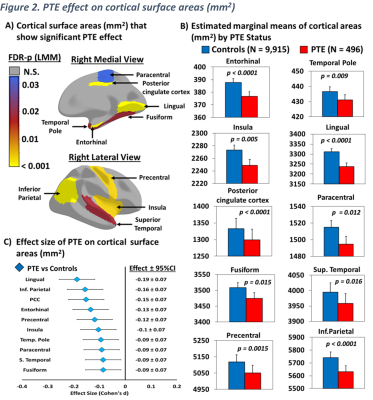
Brain morphometry (Figure 2A): Compared to unexposed children, PTE-children had smaller surface areas in the entorhinal cortex, temporal pole, insula, lingual, posterior cingulate cortex, paracentral, inferior parietal cortices, the fusiform, superior temporal, and precentral gyri (Figure 2B). Additionally, compared to unexposed children, PTE-children had smaller volumes in the amygdala, nucleus accumbens, hippocampus, globus pallidum, and the thalamus, Figure 3A and 3B. PTE effect sizes on surface areas and subcortical volumes across the brain regions remained significant after adjusting for the covariates, Figure 2C and 3C. Also, PTE had no association with cortical thickness across the selected regions.
Sex-specific effects were observed in the postcentral gyrus (interaction-FDR-p=0.015), Figure 4A-B (left), with only PTE-girls, but not PTE-boys, showing a smaller surface area compared to sex-matched controls (Figure 4B left; effect size -0.28 Figure 4C top). The posterior corpus callosum also showed sex-specific effects (interaction-p= 0.033, Figure 4A-B right); only PTE-boys (but not PTE-girls) showed a smaller volume compared to sex-matched controls (Figure 4B right; effect size 0.18, Figure 4C bottom).
Discussion
Our findings support previous neuroimaging studies showing PTE is associated with smaller subcortical grey matter volumes4,5 and poorer working memory6,7, verbal skills8 and executive function7,9. We additionally found that PTE is associated with smaller surface areas in multiple cortical regions. These novel findings may be detected because of the large sample size from the ABCD study. Previous studies also reported PTE effects in the frontal and parahippocampal regions in adolescents older than 15 years of age10,11. Therefore, the region-specific PTE effects in the current study may progress to involve frontal brain regions.PTE can expose the fetus to hypoxia due to vasoconstriction12, but also to several chemicals including nicotine in tobacco, which can cause abnormal neural development13, cell proliferation and differentiation14. Thus, smaller regional brain volumes and cortical surface areas in PTE-children suggests neurotoxic effects of PTE. A novel finding is that PTE has sex-specific effects on some brain surface areas, which earlier studies posit this may be associated with the sex-selective effect of prenatal nicotine on cholinergic and serotonergic hypoactivity and reduced neural cell membrane complexity16-18.
Limitations of the current study include: the self-reported PTE status and the cross-sectional analysis, which cannot determine a causal relationship. Future studies of longitudinal follow-ups will determine whether the possible altered neurodevelopmental trajectories will persist or may be moderated by puberty (with sex-hormones) or other familial, genetic, cultural or environmental factors.
Conclusion
Our findings suggest an association between PTE and lowered cognitive performance in children at ages 9 to 10 years old, and regional- and sex-specific decreases in cortical surface areas and subcortical volumes.Acknowledgements
We are grateful to all the research participants in the ABCD study, and all the hard work from the entire ABCD Consortium. The study was supported by funding from 11 institutes or offices at the NIH, CDC, NSF, and the NEA. The authors are supported by the Parent Grant to University of Maryland School of Medicine (U01-DA041117).References
1. Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health. 07 2018;6(7):e769-e776. doi:10.1016/S2214-109X(18)30223-7
2. Chang L, Cloak CC, Jiang CS, Hoo A, Hernandez AB, Ernst TM. Lower glial metabolite levels in brains of young children with prenatal nicotine exposure. J Neuroimmune Pharmacol. Mar 2012;7(1):243-52. doi:10.1007/s11481-011-9311-6
3. Compton WM, Dowling GJ, Garavan H. Ensuring the Best Use of Data: The Adolescent Brain Cognitive Development Study. JAMA Pediatr. Sep 01 2019;173(9):809-810. doi:10.1001/jamapediatrics.2019.2081
4. Liu J, Lester BM, Neyzi N, et al. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr. Apr 2013;167(4):348-54. doi:10.1001/jamapediatrics.2013.550
5. Haghighi A, Schwartz DH, Abrahamowicz M, et al. Prenatal exposure to maternal cigarette smoking, amygdala volume, and fat intake in adolescence. JAMA Psychiatry. Jan 2013;70(1):98-105. doi:10.1001/archgenpsychiatry.2012.1101
6. Jacobsen LK, Slotkin TA, Westerveld M, Mencl WE, Pugh KR. Visuospatial memory deficits emerging during nicotine withdrawal in adolescents with prenatal exposure to active maternal smoking. Neuropsychopharmacology. Jul 2006;31(7):1550-61. doi:10.1038/sj.npp.1300981
7. Paule MG, Rowland AS, Ferguson SA, et al. Attention deficit/hyperactivity disorder: characteristics, interventions and models. Neurotoxicol Teratol. 2000 Sep-Oct 2000;22(5):631-51. doi:10.1016/s0892-0362(00)00095-7
8. Julvez J, Ribas-Fitó N, Torrent M, Forns M, Garcia-Esteban R, Sunyer J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol. Aug 2007;36(4):825-32. doi:10.1093/ije/dym107
9. Oh K, Xu Y, Terrizzi BF, et al. Associations Between Early Low-Level Tobacco Smoke Exposure and Executive Function at Age 8 Years. J Pediatr. 06 2020;221:174-180.e1. doi:10.1016/j.jpeds.2019.11.032
10. Lotfipour S, Ferguson E, Leonard G, et al. Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry. Nov 2009;66(11):1244-52. doi:10.1001/archgenpsychiatry.2009.124
11. Toro R, Leonard G, Lerner JV, et al. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. Apr 2008;33(5):1019-27. doi:10.1038/sj.npp.1301484
12. El Marroun H, Schmidt MN, Franken IH, et al. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology. Mar 2014;39(4):792-800. doi:10.1038/npp.2013.273
13. Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. Jun 2001;40(6):630-41. doi:10.1097/00004583-200106000-00007
14. Slotkin TA, Greer N, Faust J, Cho H, Seidler FJ. Effects of maternal nicotine injections on brain development in the rat: ornithine decarboxylase activity, nucleic acids and proteins in discrete brain regions. Brain Res Bull. Jul 1986;17(1):41-50. doi:10.1016/0361-9230(86)90159-0
15. PR. H. Neural plasticity: the effects of environment on the development of the cerebral cortex. Harvard University Press; 2002
16. Navarro HA, Seidler FJ, Eylers JP, et al. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther. Dec 1989;251(3):894-900.
17. Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. Jul 15 2004;198(2):132-51. doi:10.1016/j.taap.2003.06.001
18. Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. May 2007;32(5):1082-97. doi:10.1038/sj.npp.1301231
Figures
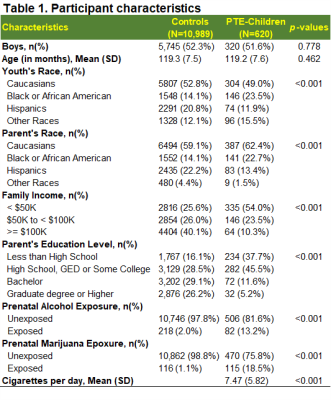
A) Displays estimated marginal means by PTE status of neurocognitive measures that showed significant PTE effect, calculated using linear mixed effects models and emmeans (p-values are from post hoc group comparisons). B) Effect size (Cohen’s d) and 95% confidence Intervals of PTE for neurocognitive measures shown in (A), calculated using emmeans.
A) Displays cortical surface areas that showed a significant PTE effect after adjusting for covariates, calculated using linear mixed models (FDR-p: p-values corrected for multiple comparison using false discovery rate), B) displays the estimated marginal means by PTE status of surface areas shown in (A), calculated using linear mixed effects models and emmeans (p values are from post hoc group comparisons), C) Effect size (Cohen’s d) and 95% confidence Intervals of PTE for surface areas shown in (A and B), calculated using emmeans.
A) Displays subcortical volumes that showed a significant PTE effect after adjusting for covariates, calculated using linear mixed models (FDR-p: p-values corrected for multiple comparison using false discovery rate), B) Estimated marginal means by PTE status of subcortical volumes shown in (A), calculated using linear mixed effects models and emmeans (p values are from post hoc group comparisons), C) Effect size (Cohen's d) and 95% confidence Intervals of PTE for subcortical volumes shown in (A and B), calculated using emmeans.
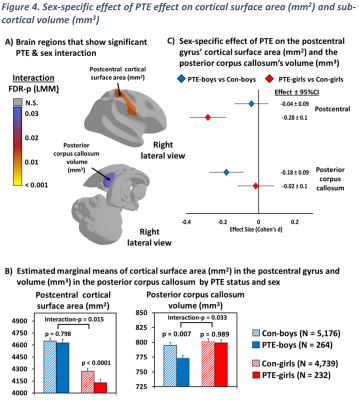
A) Shows regions that showed a significant PTE and sex interaction after adjusting for covariates, calculated using linear mixed model (interaction-p values were corrected for multiple comparison using false discovery rate), B) Displays estimated marginal means by PTE status & sex for regions shown in (A), calculated using linear mixed effects models & emmeans (Interaction-p from LMM; p-values from post hoc group comparisons), C) Effect size (Cohen's d) and 95% confidence Intervals of PTE by sex group for regions shown in (A and B), calculated using emmeans.