2727
Fabrication of an Anthropomorphic, In-Vitro Liver Flow Phantom for Use in Motion-Robust MRI Sequence Validation Experiments
James Rice1,2, Ruiqi Geng2,3, Diego Hernando2,3,4,5, and Alejandro Roldan-Alzate1,2
1Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Electrical and Computer Engineering, University of Wisconsin-Madison, Madison, WI, United States, 5Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
1Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Electrical and Computer Engineering, University of Wisconsin-Madison, Madison, WI, United States, 5Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
A major limitation of standard diffusion-weighted imaging (DWI) in the various organs, including the liver, is the presence of artifacts, bias, and poor reproducibility resulting from physiologic cardiovascular-induced motion. Recent efforts to develop motion-robust DWI sequences show promise, but there is an important unmet need for highly controlled validation in motion phantoms. This study presents the fabrication of an anthropomorphic liver flow phantom that can produce compressive motion in the liver to generate and control artifact seen in DW images. This phantom may enable improved validation of motion-robust DWI and other motion-robust applications.
Introduction
The development of motion-robust MRI methods continues to be an active area of MR research. Motion-robust methods are particularly important in diffusion-weighted imaging (DWI) of the abdomen (including the liver). Importantly, non-rigid tissue movement induced by cardiac pulsation or blood perfusion results in substantial artifacts, bias and poor reproducibility in DWI1–3. Recently developed motion-robust DWI methods may enable reliable evaluation of the liver, with low bias and excellent reproducibility2,4,5. However, validation of these methods has been limited, and evaluation in anatomically accurate phantoms with controlled motion remains an unmet need. Hydrogel models have proved useful in creating tissue-like phantoms that can be imaged with MRI3. Pulsatile in-vitro flow phantoms allow for systematic control of flow in complex geometries and can produce physiologic flow profiles6-7. This study combined these two methodologies and presents a framework for fabricating anthropomorphic, hydrogel-based liver phantoms capable of reproducing highly controlled compressive motion in the liver and extract ground-truth images that can be used to enable the development and optimization of motion-robust MRI methods.Methods
An anthropomorphic, in-vitro, MR-compatible model of the liver was constructed using a hybrid additive manufacturing process6. The liver was segmented from a computed tomographic (CT) dataset of a healthy adult and used to generate a stereolithography (STL) file which was imported to 3MATIC (Materialise) where it was manipulated to produce a hollow liver mold. and 3D printed using a stereolithography printer (SLA; Formlabs). Space was left for a bifurcation representing an idealized portal vein. The bifurcation was 3D printed using water-soluble polyvinyl alcohol (PVA) and coated with latex. The PVA core was dissolved leaving a hollow vessel which could be connected to a flow circuit. The pseudo-tissue liver was constructed using hydrogel material base solution of two polymer components -acrylamide and bis-acrylamide (Fisher Scientific) which resulted in a gel composition of 8% and elastic modulus of 50kPa. The solution was then poured inside the mold and cured, producing a distensible liver around the bifurcation (Figure 1). The model was connected to a MRI compatible pulsatile flow circuit (BDC Laboratories) where flow rate and cardiac waveform could be controlled to alter hemodynamics in the bifurcation. 3.8L of distilled water was doped with 4g of gadobenate dimeglumine to achieve T1 of ~230ms and T2 of ~92ms, close to human tissue. The phantom was scanned on a 3.0T MRI system (Signa Premier, GE) with high density receive array coils (AIR coil, GE). To visualize motion, cine images of the model were acquired throughout the flow cycle. To evaluate motion-induced artifacts in the model, imaging was performed using DWI with standard monopolar (MONO, TE = 49ms) diffusion waveforms, with orthogonal diffusion directions were obtained at two different b-values (b=50,500s/mm2).Results
Figure 1 outlines the model creation process. The STL and physical models are depicted in the top and bottom rows, respectively. Figure 2 shows axial and coronal cine images of phantom motion during flow experiments. Pulsatile motion in the tube is seen as it contracts and expands over the cardiac cycle. The periodic nature of the flow profile generates pulsatility in the tube which translates to compressive motion in the liver as it enlarges and contacts the phantom. The motion is observed adjacent to the tube and seen in both views of the model. Additionally, the rigid walls allow for reflection of compressive motion as waves travel from the tube-model interface to the periphery of the liver. Figure 3 includes DW images of the liver phantom under a nominal flow of 1.3L/min. Compressive-tissue-induced artifacts appear adjacent to the bifurcation; artifact severity worsens at higher b-value. This results in skewed values of apparent diffusion coefficient, which is a measure of water diffusion in tissues. This effect closely mimics the artifacts observed in DWI of the liver and other organs2,3,5. A co-localized T2-weighted image was shown for geometric reference.Discussion
This study presents a novel approach to the construction and use of an anthropomorphic, distensible flow phantom for simulating tissue motion in the liver. This phantom produced controlled compressive motion and DWI artifacts that closely resemble those observed in vivo, highlighting the phantom’s ability to produce physiologic motion. Motion was achieved near the bifurcation, where motion artifact is observed in the DW images. As flow amplitude increases, compressive motion induced from the tube increases. Through manipulation of the pump flow amplitude, motion in the liver is controlled. If desired, geometry and orientation of the tube can be altered to produce compressive motion patterns. Additionally, the hydrogel liver was fabricated with a tissue stiffness corresponding to a modulus within the range of results for liver tissue8. Stiffness properties can be manipulated by adjusting the gel composition, either producing a stiffer or more distensible tissue. Future efforts aim at adjusting stiffness as needed to expand the scope of DWI validation.Conclusion
This study outlined the fabrication and use of an anthropomorphic liver phantom capable of simulating compressive tissue motion that can be imaged with DWI MR. Repeatable motion in the model induced artifact in DW images, mimicking effects observed in vivo. Use of similar phantoms shows promise to enable highly controlled validation of motion-robust DWI in the liver and other organs.Acknowledgements
The authors acknowledge support from the NIH (R01 EB030497) as well as GE Healthcare, who provides research support to the University of Wisconsin-Madison.References
- Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254(1):47-66. doi:10.1148/radiol.09090021
- Zhang Y, Peña-Nogales Ó, Holmes JH, Hernando D. Motion-robust and blood-suppressed M1-optimized diffusion MR imaging of the liver. Magn Reson Med. 2019;82(1):302-311. doi:10.1002/mrm.277353.
- Geng R, Zhang Y, Starekova J, et al. Characterization and correction of cardiovascular motion artifacts in diffusion-weighted imaging of the pancreas. Magn Reson Med. 2021;86(4):1956-1969. doi:10.1002/mrm.288464.
- Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI. Magn Reson Med. 2017;77(2):717-729. doi:10.1002/mrm.261665.
- Peña-Nogales Ó, Zhang Y, Wang X, et al. Optimized Diffusion-Weighting Gradient Waveform Design (ODGD) formulation for motion compensation and concomitant gradient nulling. Magn Reson Med. 2019;81(2):989-1003. doi:10.1002/mrm.274626.
- Rutkowski DR, Kim C, Roldán-Alzate A. Hydrogel-based phantoms for magnetic resonance imaging. Proc Summer Biomech Bioeng Biotransport Conf. (SB3C2020) #376.
- Rutkowski D, Medero R, Ruesink T, et al. Modeling physiological flow in Fontan models with four-dimensional flow magnetic resonance imaging, particle image velocimetry, and arterial spin labeling. J Biomech Eng. 2019;141(12):121004.
- Nava A, Mazza E, , Furrer M, et al. In vivo mechanical characterization of the human liver. Medical Image Analysis. 2006;12(2):203-216. doi: 10.1016/j.media.2007.10.001.
Figures
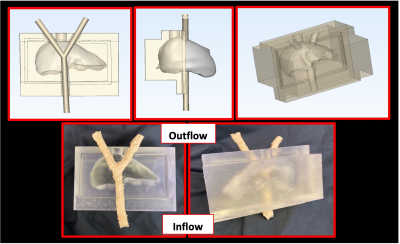
Figure 1: Pipeline for constructing anthropomorphic, in-vitro, MRI-compatible hydrogel flow model of the liver. Top row depicts slices of the STL model used to fabricate physical model (bottom). When connected to pulsatile flow circuit, flow enters the model through the single branch shown at the bottom of the model and exits out of the two top branches where pulsation from inner tube drives compressive motion in hydrogel.
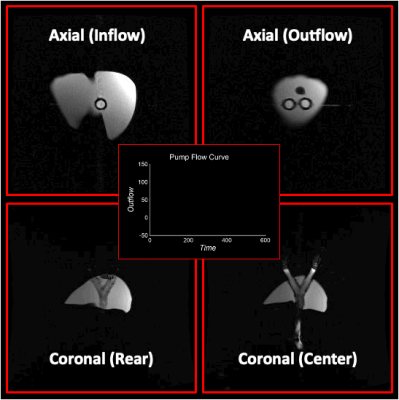
Figure 2: Visualization of motion in the pseudo-tissue liver induced from pulsating inner tube in axial (top) and coronal (bottom) views of the model. Water was pumped through the flow circuit where the flow profile was controlled to achieve pulsatile flow with a mean flow rate of 1.3 L/min (center). Clear pulsation of the bifurcation tube is seen in both views. Compressive motion from tube induces deformation of liver in regions near the contact point of the liver and tube.
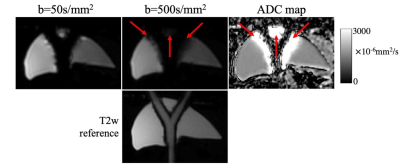
Figure 3: Diffusion-weighted images acquired with b=50, 500s/mm2 and the map of apparent diffusion coefficient (ADC) calculated from the diffusion-weighted signals. At high degree of compressive tissue motion induced by pulsatile motion, signal voids due to phase variations appear in the high-b-value images, resulting in biased ADC values around the tubing (indicated by red arrows). A co-localized T2-weighted image was shown for geometric reference. These results closely mimic the artifacts observed in vivo, but in a highly controllable setting.
DOI: https://doi.org/10.58530/2022/2727