2725
Soy lecithin: a phantom material for the adjustment of apparent diffusion coefficient in magnetic resonance imaging1Department of Diagnostic and Interventional Radiology, University of Tuebingen, Germany., Section on Experimental Radiology, Tuebingen, Germany, 2Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Centre Munich at the University of Tuebingen, Tuebingen, Germany
Synopsis
The aim of this work was to systematically investigate whether lecithin is suitable for the construction of a diffusion phantom that covers and simulates ADC-values occurring in human tissue. For this, aqueous solutions of different lecithin concentrations (0%-10%) were prepared and measured by DWI. The presented results showed that lecithin is a suitable agent for simulating a wide variety of ADC-values. Even low concentrations showed a strong decrease in the ADC-value of water. However, with increasing concentration, lecithin also showed a strong influence on T2, so that ADC and T2 cannot be set independently from one another in lecithin-based phantoms.
Introduction
The apparent diffusion coefficient (ADC), as determined from diffusion weighted imaging (DWI), plays a crucial role in diagnosis and assessment of a variety of disorders1-3. With the advent of new imaging techniques and new MRI systems, the development of suitable phantoms simulating ADC values of tissue is becoming more and more important. The determination of quantitative MR parameters should be as consistent as possible in examinations on different MRI systems. Only then are absolute values and results of follow-up examinations of patients on different MRI systems comparable. Reliable measurement phantoms are therefore indispensable for testing MRI equipment. For simulation of ADC values of tissue, some phantoms have already been proposed, which are mostly based on sucrose, agarose gels or polyethylene glycol (PEG)4-7. A large amount of substance is usually needed to simulate small ADC values and some of these substances show interfering signals in MRI experiments. Recently, we have identified soy lecithin as an attractive agent for adjusting diffusion properties8. Soy lecithin is a natural occurring emulsifier that is mainly used in the food industry. The aim of this work was to systematically investigate whether lecithin is suitable for the construction of an MR diffusion phantom that covers and simulates ADC values occurring in human tissue. In addition, the influence of lecithin on the transverse relaxation time (T2) of water was investigated.Materials and Methods
Data acquisition and analysisImaging was performed on a clinical-, whole-body 3.0 T MR system (MAGNETOM Prismafit, Siemens Healthcare, Erlangen, Germany) using a standard 64-channel head coil. All data were collected at room temperature (approx. 22 °C) and processed offline with MATLAB (MathWorks, Inc., Natick, MA).
Sample preparation
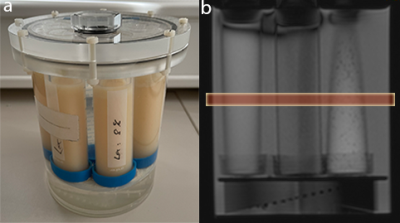
To check the effects of soy lecithin (purchased from Carl Roth, Karlsruhe, Germany) on diffusivity and transverse relaxation time (T2) of water, aqueous solutions of varying concentrations were prepared in volumes of 50 ml. The concentration of dissolved lecithin ranged from 0 % to 10 % with respect to the aqueous phase. To increase solubility, the aqueous phase was heated up to 30 – 40 °C using a water bath. It has also been shown that the use of a mortar and pestle makes it easier to completely dissolve the agent in the aqueous phase. The total preparation time for all 10 solutions was approximately 90 minutes. The cost of the agent is around 20-30 euros per 250 grams. The solutions were stored in CELLSTAR polypropylene tubes (Greiner Bio-One, Frickenhausen, Germany) and fixed in a cylindrical MRI phantom (Figure 1a). Since the measurement phantom did not offer space for all samples, the measurements were carried out twice: first for the samples with a concentration of 1 % - 5 % and then for the samples with the higher concentrations (6 % - 10%).
Influence of soy lecithin on the diffusivity of water
The effect of soy lecithin on the ADC value of water was investigated by diffusion-weighted imaging. DWI was performed using a readout-segmented echo planar imaging sequence in 3-scan-trace mode with monopolar diffusion gradients, four different b-values (0, 50, 500, 1000), and coronal slice orientation (Figure 1b). TR and TE were set to 3000 ms and 51 ms, respectively. ADC-maps were calculated from the acquisitions with multiple b-values using a log-linear fitting of the signal intensities and ADC values were determined from extended regions of interest (ROI) within each sample.
Influence of soy lecithin on the transverse relaxation time of water
Relaxometric measurements were performed to investigate whether and to what extent the presence of lecithin might also modify the transverse relaxation time of water. For this purpose, quantitative T2 maps were acquired using a Carr-Purcell-Meiboom-Gill (CPMG) spin-echo pulse sequence with a TR of 6000 ms and 32 different TEs. The initial and final TE were set to 50 ms and 1600 ms (increment: 50 ms). A three-parameter model was used for pixelwise monoexponential fitting of the measured signal intensities. In analogy to the measurements of the ADC values, T2 was determined in extended ROIs within each sample.
Results and Discussion
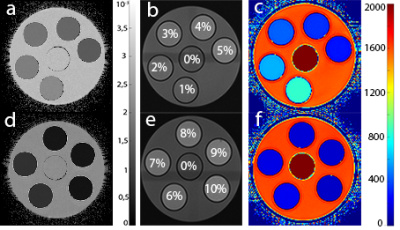
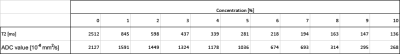
Calculated ADC- and T2 maps are shown in Figure 2. Table 1 summarizes the ADC- and T2 values of water as a function of the concentration of dissolved lecithin. Both the ADC value and T2 show a strong decrease with increasing concentration. These findings show that lecithin is a suitable substance to simulate a wide range of ADC values with low resource consumption. The distinct decrease in ADC values and the fact that lecithin does not show any artifacts in the images makes lecithin particularly interesting for the construction of DWI phantoms. Nevertheless, the simultaneous strong decrease in T2 must be viewed critically, as this means that ADC and T2 cannot be set independently of one another in lecithin-based diffusion phantoms.Conclusion
This work shows that soy lecithin is a suitable agent for simulating a wide variety of ADC values. Even low concentrations of lecithin showed a strong decrease in the ADC value of water. However, the fact that lecithin also has a strong influence on T2 can, depending on the objective, be a hindrance and must be taken into account when lecithin is used as a diffusion phantom.Acknowledgements
This work was supported and funded by the German Research Foundation (DFG) under Grants SCHI 498/14-1 (Package No. 997/1).References
1: Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006 Jun;239(3):632-49. doi: 10.1148/radiol.2393042031. PMID: 16714455.
2: Fung SH, Roccatagliata L, Gonzalez RG, Schaefer PW. MR diffusion imaging in ischemic stroke. Neuroimaging Clin N Am. 2011 May;21(2):345-77, xi. doi: 10.1016/j.nic.2011.03.001. PMID: 21640304.
3: van Houdt PJ, Yang Y, van der Heide UA. Quantitative Magnetic Resonance Imaging for Biological Image-Guided Adaptive Radiotherapy. Front Oncol. 2021 Jan 29;10:615643. doi: 10.3389/fonc.2020.615643. PMID: 33585242; PMCID: PMC7878523.
4: Gatidis S, Schmidt H, Martirosian P, Schwenzer NF. Development of an MRI phantom for diffusion-weighted imaging with independent adjustment of apparent diffusion coefficient values and T2 relaxation times. Magn Reson Med. 2014 Aug;72(2):459-63. doi: 10.1002/mrm.24944. Epub 2013 Oct 2. PMID: 24123316.
5: Laubach HJ, Jakob PM, Loevblad KO, Baird AE, Bovo MP, Edelman RR, Warach S. A phantom for diffusion-weighted imaging of acute stroke. J Magn Reson Imaging. 1998 Nov-Dec;8(6):1349-54. doi: 10.1002/jmri.1880080627. PMID: 9848751.
6:Matsuya R, Kuroda M, Matsumoto Y, Kato H, Matsuzaki H, Asaumi J, Murakami J, Katashima K, Ashida M, Sasaki T, Sei T, Himei K, Katsui K, Katayama N, Takemoto M, Kanazawa S, Mimura S, Oono S, Kitayama T, Tahara S, Inamura K. A new phantom using polyethylene glycol as an apparent diffusion coefficient standard for MR imaging. Int J Oncol. 2009 Oct;35(4):893-900. doi: 10.3892/ijo_00000404. PMID: 19724927.
7: Lavdas I, Behan KC, Papadaki A, McRobbie DW, Aboagye EO. A phantom for diffusion-weighted MRI (DW-MRI). J Magn Reson Imaging. 2013 Jul;38(1):173-9. doi: 10.1002/jmri.23950. Epub 2013 Apr 10. PMID: 23576443.
8: Fritz V, Martirosian P, Machann J, Daniels R, Schick F. A comparison of emulsifiers for the formation of oil-in-water emulsions: stability of the emulsions within 9 h after production and MR signal properties. MAGMA. 2021 Oct 26. doi: 10.1007/s10334-021-00970-9. Epub ahead of print. PMID: 34698962.
Figures