2724
Investigation of sodium alginate- and polyvinyl alcohol-based anisotropic hydrogel phantoms for calibration applications by DWI at 0.6 T1Faculty of Physics and Applied Computer Science, AGH University of Science and Technology, Kraków, Poland, 2Faculty of Geology, Geophysics and Environmental Protection, AGH University of Science and Technology, Kraków, Poland, 3Faculty of Materials Science and Ceramics, AGH University of Science and Technology, Kraków, Poland
Synopsis
Anisotropic hydrogels has been attracting attention for biomedical purposes. These highly-hydrated materials have potential for tissue-mimicking and then, may serve for advanced medical imaging studies. In the work, we present two types of anisotropic hydrogels made from sodium alginate (NA) and polyvinyl alcohol (PVA) in the form of fiber bundle- a single component for larger phantom. We investigated their potential for diffusion-weighted imaging. NA-based phantoms are characterized by highly uniform diffusivity distribution, even higher then water. Single component turned out to be insufficient for the accurate quantification of the microstructure. PVA-based phantom exhibited desirable diffusion properties for tissue-mimicking.
INTRODUCTION
Hydrogels are highly hydrated polymers that has been widely applied in biomedical engineering, in the isotropic and anisotropic form as medium for cell culture or tissue engineering and potential use as tissue substitute [1]. In recent years, anisotropic hydrogels attracted attention in biomedical sciences, namely as promising material to mimic anisotropic tissues [1]–[5]. Realistic, tissue-mimicking phantoms, as well as periodic anisotropic phantoms are essential for calibration procedures in medical imaging and protocol testing [2],[6]–[8]. Previously, we presented several types of anisotropic phantoms with well-defined, capillary and planar structures and their significant role in magnetic gradient field calibration in diffusion-weighted (DWI) and diffusion tensor imaging (DTI) [6],[8],[9]. Although meaningful and innovative, they bear a major drawback of advanced technology requirement for their manufacturing from SiO2 and photonic fibers. This imposes very high production costs. Here, we present a prototype of anisotropic, biopolymer-based and polyvinyl alcohol-based phantoms for magnetic resonance imaging applications. We investigated diffusion properties of polymeric fiber bundle constituting a single component for a large, tissue-mimicking (e.g. nervous or muscle) anisotropic phantom for the purpose of DWI/DTI calibration and also for other MRI modalities.METHODS
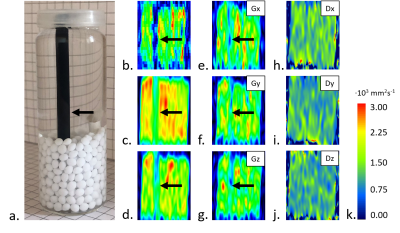
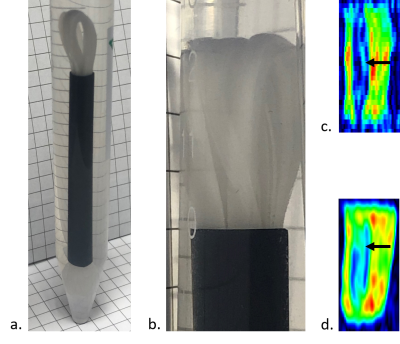
Sodium alginate (NA; NovaMatrix, Sandvika, Sweden) was chosen based on the test DWI experiments on isotropic hydrogels samples (images not shown). Second phantom was made from polyvinyl alcohol (PVA). Thin fibers were then obtained by wet-drawing method from 2%wt. NA crosslinked with calcium chloride and 6 wt.% polyvinyl alcohol (VRM International PTE.LTD, India). Directional fiber alignment was obtained by inserting a fiber bundle into a polypropylene tube to achieve tight filling. The model was filled with ethyl alcohol (96%, POCh). Biopolymer fibers made from NA showed smaller single fiber diameter (10-12 μm) and lower material stiffness (E=0.54 MPa) than PVA fibers (fiber diameter of 15-18 μm and E=1.82 MPa). The degree of crosslinking for biopolymer and PVA fibers equal to 40-60% and 65%, respectively. Parallel arrangement of the fiber bundle allows the diameter of the tube (to 92%) into which the medium in the form of ethanol has been infiltrated to be filled for better durability of the fiber systems. DWI was conducted on 0.6 T laboratory tomography system (Magritek, Aachen, Germany) using spin-echo two-dimensional DWI in three orthogonal directions x, y, z (repetition time/echo time, TR/TE=10 s/56 ms; number of scans, NoS=16 and FOV=40x60 mm2 for NA-based and NoS=8 and FOV=40x100 mm2 for PVA-based phantoms; slice thickness, ST= 2 mm; matrix=128x128; b-value=1000 s mm-1).RESULTS
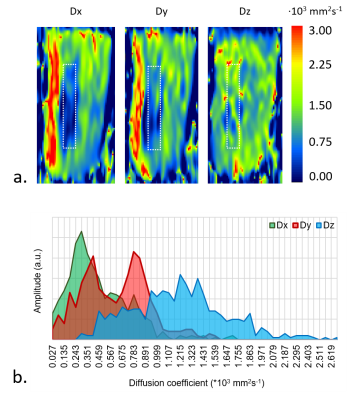
We tested series of isotropic hydrogel samples and chose 1% NA in the water medium, since having uniform and almost as high as water’s diffusion coefficient (diffusion coefficients of water and 1% NA hydrogel were Dwater=2.44±0.11∙10-3 mm2s-1 and D1% NA=2.272±0.059∙10-3 mm2s-1, respectively). NA-based component was very thin and exhibited poor anisotropic properties in conducted DWI experiments (longitudinal diffusivity did not stand out; Table 1, Fig. 2). PVA-based phantom (Fig. 2) had significantly higher cross-section diameter and revealed visibly demarked longitudinal (D||,PVA=Dz) and transversal component (D⊥,PVA=0.5(Dx+Dy); Table 1, Fig. 2).DISCUSSION
Isotropic sample of 1% NA-water solution showed excellent diffusion coefficient uniformity in the measured volume, slightly above two times higher than water. DWI was restricted for anisotropic NA hydrogel by the very low resolution needed to capture such small diameter of fiber bundle (Fig. 1). Thicker bundle or joined bundles will be further considered. In PVA hydrogel D||,PVA/D⊥,PVA≈2.3 which meets the requirements for similarity to tissues. Double mode in Dy may result from the placement method in the black tube (bundle folded in half).CONCLUSION
Presented anisotropic hydrogels delivered preliminary information on their microstructure visible in DWI. PVA-based phantom is very stable in time and exhibit desirable diffusive properties. Considering very high resolution used for NA-based phantom, it delivered reasonable data. Whereas needs some manufacturing improvement, NA material seems to be promising material for tissue-mimicking phantom with uniform properties. Presented data will serve for manufacturing methodology enhancement in order to approach an optimal setting for clinical applications.Acknowledgements
The work was financed by the Medical Research Agency (Contract No. 2020/ABM/01/00006-00). W.M. has been partly supported by the EU Project POWR.03.02.00-00-I004/16.References
1. Sano K, Ishida Y, Aida T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew Chemie Int Ed. 2018;57(10):2532-2543. doi:10.1002/ANIE.201708196
2. Chatelin S, Breton E, Arulrajah A, et al. Investigation of PolyVinyl Chloride Plastisol Tissue-Mimicking Phantoms for MR- and Ultrasound-Elastography. Front Phys. 2020;8:522. doi:10.3389/FPHY.2020.577358/BIBTEX
3. Hunold A, Machts R, Haueisen J. Head phantoms for bioelectromagnetic applications: a material study. Biomed Eng Online. 2020;19(1):1-14. doi:10.1186/S12938-020-00830-Y/FIGURES/8
4. Jian Y, Handschuh-Wang S, Zhang J, Lu W, Zhou X, Chen T. Biomimetic anti-freezing polymeric hydrogels: keeping soft-wet materials active in cold environments. Mater Horizons. 2021;8(2):351-369. doi:10.1039/D0MH01029D
5. Guidetti M, Lorgna G, Klatt D, Vena P, Royston TJ. Anisotropic composite material phantom to improve skeletal muscle characterization using magnetic resonance elastography. J Mech Behav Biomed Mater. 2019;89:199-208. doi:10.1016/J.JMBBM.2018.09.032
6. Kłodowski K, Krzyżak AT. Innovative anisotropic phantoms for calibration of diffusion tensor imaging sequences. Magn Reson Imaging. 2016;34(4):404-409. doi:10.1016/j.mri.2015.12.010
7. Borkowski K, Kłodowski K, Figiel H, Krzyżak AT. A theoretical validation of the B-matrix spatial distribution approach to diffusion tensor imaging. Magn Reson Imaging. 2017;36:1-6. doi:10.1016/j.mri.2016.10.002
8. Mazur W, Krzyżak AT, Raszewski Z. Towards the precise microstructural mapping. Testing new anisotropic phantoms with layered and capillary geometries. In: 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. ; 2019:2835-2839. doi:10.1109/EMBC.2019.8857065
9. Krzyżak AT. Anisotropic diffusion phantom for calibration of any MR, DTI imaging sequence and method of calibration of any MR tomograph. Published online 2008:30-31.
Figures