2719
Simultaneous imaging of positive and negative chemical exchange contrast: On the implementation of the RACETE-technique on a clinical 3T-system1Experimental Physics 5, University of Würzburg, Würzburg, Germany, 2Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany, 3Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben, Germany, 4Magnetic Resonance and X-ray Imaging Department Fraunhofer IIS, Fraunhofer Institute for Integrated Circuits IIS, Würzburg, Germany
Synopsis
Contrary to the basic principle of CEST, the RACETE technique allows for the direct detection of positive chemical exchange contrast. This method, which was previously demonstrated only under ultra-high field conditions at 7T-17.5T, has now successfully been implemented on a clinical 3T scanner in initial phantom experiments. Furthermore, we present a novel dual-contrast RACETE-technique for simultaneous imaging of the positive RACETE and the negative CEST contrast.
Introduction
Chemical exchange saturation transfer (CEST) provides an indirect measurement of dilute labile protons by probing their chemical exchange (CE) with bulk water protons via transfer of saturated magnetization [1,2]. Since CE effects are ubiquitous in nuclear magnetic resonance, CEST imaging has been applied in numerous studies focusing on clinical molecular imaging over the past decade [3]. Contrary to the basic principle of CEST, the RACETE (Refocused Acquisition of Chemical Exchange Transferred Excitations) technique allows for the direct measurement of positive CE contrast and eliminates the necessity of additional reference scans [4].RACETE is based on a stimulated-echo-technique using a train of solute-selective excitation-transfer modules (ETMs) and requires precise implementation, preferably at high field strengths. Imaging of true positive CE contrast with RACETE has already been demonstrated under ultra-high field conditions (17.5T) [4]. This study shows the feasibility of RACETE on a standard clinical 3T scanner. Furthermore, we present a method for rapid simultaneous imaging of positive and negative CE contrast using interleaved spiral acquisitions.
Sequence design
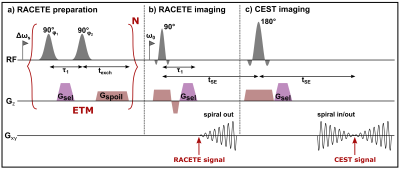
The sequence diagram for RACETE is shown in Figure 1 [4]. A single excitation-transfer module (ETM) consists of two gaussian 90° pulses, selective (Δωs) on the solute protons (Fig. 1a). During the τ1 delay, between the two solute-selective pulses, a dephasing selection gradient Gsel is applied, which labels the dilute spins with a specific phase. In each ETM cycle solute protons are excited, labeled and stored as longitudinal magnetization. The excitation cycles are followed by a stimulated-echo acquisition (Fig. 1b) via selective refocusing of exchanged solute protons now present in the solvent pool. Here, the same selection gradient is applied for rephasing the accumulated transferred spins. At the same time Gsel suppresses the free induction decay of bulk water spins which have not exchanged during the preparation. In this way, RACETE generates positive CE contrast and suppresses background signals of bulk water. Furthermore, a CEST-like signal can be acquired in direct succession by refocusing the magnetization to a second stimulated echo (Fig. 1c). Thus, positive RACETE and negative CEST-type contrast can be measured simultaneously within a single preparation. In contrast to the original sequence [4], a spiral readout is employed for both.Methods
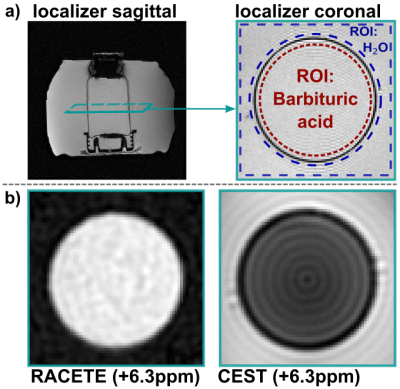
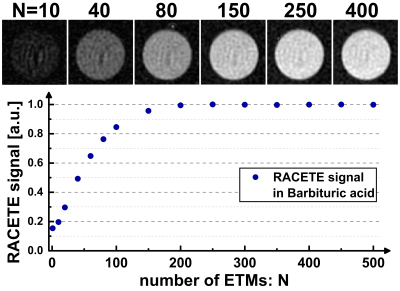
The RACETE sequence was implemented on a clinical 3T scanner (MAGNETOM Skyra, Siemens, Erlangen, Germany) within the open-source Pulseq framework [5,6]. As a special feature, the imaging of RACETE and CEST signals was realized using interleaved spiral acquisitions. For initial experiments Barbituric acid (concentration 100mmol/l, pH=3.3) solved in water and sodium hydroxide solution served as a phantom with chemical exchange property whose parameters (for example exchange rate) fit the requirements for the RACETE method. The 250ml bottle was placed inside a container filled with water only.The novel dual-contrast method was successfully validated in a simple phantom experiment (Fig. 2a). In addition, RACETE and CEST-weighted images were acquired simultaneously for different chemical shifts (-10…10ppm) to obtain an overall spectrum. For a single image, four preparations each with one spiral interleave were carried out (FOV: 230x230mm2, slice: 15mm, matrix: 96x96, 250 ETMs, τ1=6.5ms, texch=17ms, recovery time 5s). The contrast evaluation was done in two ROIs (region-of-interest, Fig. 2b). Furthermore, the number of ETMs has been varied to study the influence on the positive RACETE contrast.
Results
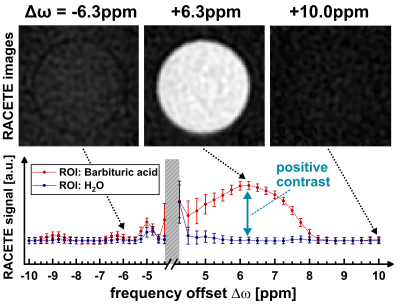
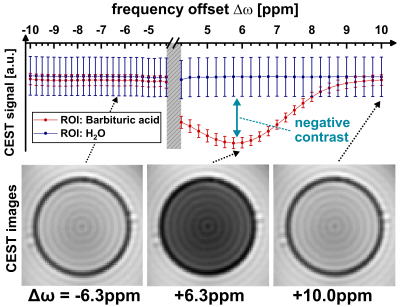
Figure 2b demonstrates the generation of true positive CE contrast at a chemical shift of +6.3ppm using the RACETE technique. The generated negative contrast for the additional CEST-weighted image within the same preparation can be clearly seen in Fig. 2b.Figure 3 and 4 show the RACETE and CEST intensity spectra, respectively. While the RACETE spectrum provides a clear positive contrast in the range of 5…7ppm, the corresponding CEST spectrum shows a decrease in intensity in the same range. However, contrary to the CEST-weighted images, the RACETE images show a complete suppression of water signal.
In Figure 5, one can see that the signal increases monotonically with the number N of preparation cycles until it reaches a steady state. Starting at around 250 ETMs, the T1 relaxation during texch counterbalances the accumulation of the additional magnetization.
Discussion
Our initial phantom experiments prove the feasibility of RACETE on clinical MRI systems at 3T and the results obtained so far are in good agreement with high field experiments [4]. Despite the smaller frequency offset at 3T, positive contrast could be successfully detected. The fast spiral acquisition and the simultaneous measurement of a negative reference contrast pave the way for potential in vivo applications. Furthermore, translating RACETE to clinical 7T systems, which offer significantly higher chemical shift, can be realized quickly in the MR community thanks to the open-source Pulseq-based implementation.Acknowledgements
This work was supported by the Bundesministerium für Bildung und Forschung (BMBF Projekt 01EO1504).References
[1] Zaiss M, Bachert P. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theoretical approaches and methods. Phys Med Biol. 2013 Nov 21;58(22):R221-69. doi: 10.1088/0031-9155/58/22/R221
[2] Ji Y, et al. Progress toward quantitative in vivo chemical exchange saturation transfer (CEST) MRI. Isr J Chem. 2017. 57, 809. doi: 10.1002/ijch.201700025
[3] Jones KM, Pollard AC, Pagel MD. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J Magn Reson Imaging. 2018 Jan;47(1):11-27. doi: 10.1002/jmri.25838
[4] Gutjahr FT, et al. Positive chemical exchange contrast in MRI using Refocused Acquisition of Chemical Exchange Transferred Excitations (RACETE). Z Med Phys. 2019 May;29(2):184-191. doi: 10.1016/j.zemedi.2018.05.005
[5] Layton KJ, et al. Pulseq: A rapid and hardware-independent pulse sequence prototyping framework. Magn Reson Med. 2017 Apr;77(4):1544-1552. doi: 10.1002/mrm.26235
[6] Herz K, et al. Pulseq-CEST: Towards multi-site multi-vendor compatibility and reproducibility of CEST experiments using an open-source sequence standard. Magn Reson Med. 2021 Oct;86(4):1845-1858. doi: 10.1002/mrm.28825
Figures