2710
On the Prospects of Magnetization Transfer Imaging at 0.55T1Division of Radiological Physics, Department of Radiology, University Hospital Basel, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland
Synopsis
Magnetization transfer (MT) imaging has been extensively used to explore microstructural changes in the brain at high fields. In this work, we explore the potential of a 3D half-radial dual-echo balanced steady-state free precession (bSSFP) sequence for fast whole-brain magnetization transfer ratio (MTR) imaging at low-field strength. Our work indicates superiority of MT-sensitized bSSFP against conventional MT-prepared spoiled gradient echo (SPGR) in terms of MT contrast and resolution within similar scan time. In conclusion, MTR imaging with bSSFP offers excellent prospects for broad clinical translation and application at low fields.
Introduction
Magnetization transfer (MT) imaging, reflecting the exchange of magnetization between mobile and bound protons, has shown excellent sensitivity to detect microstructural changes in the brain caused by aging and diseases [1,2]. Frequently, a spoiled gradient echo (SPGR) sequence is used to explore the MT contrast using pulsed off-resonance irradiation. Alternatively, MT contrast can also be explored by balanced steady-state free precession (bSSFP) from an adaptation of the RF pulse duration (TRF) [3]. In contrast to MT-prepared SPGR, MT-sensitized bSSFP imaging is considerably faster but can be hampered at high fields by the presence of strong susceptibility related off-resonances. In this work we thus explore the potential of high-resolution whole-brain bSSFP-MT imaging on a low-cost commercial 0.55T clinical system.Methods
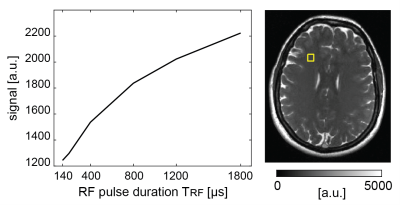
Imaging was performed on a commercially available 0.55T low-field MR-system (MAGNETOM Free.Max, Siemens Healthineers, Erlangen, Germany) using a 12-channel head coil. For MT-sensitized bSSFP brain imaging, a 3D half-radial dual-echo bSSFP sequence (termed bSTAR) [4] was used. Imaging was performed with a flip angle of 40°, 775Hz/px bandwidth, 40’000 radial half-projections, 208 samples per half-projection for a field-of-view (FOV) of 256x256x256mm3, yielding maximal isotropic resolution of 0.86mm. MT contrast was modulated by a modulation of the non-selective RF pulse duration from 0.14ms to 1.8ms (TR/TRF = 2.9/0.14ms, 2.9/0.2ms, 3.1/0.4ms, 3.5/0.8ms, 3.9/1.2ms, 4.5/1.8ms) with acquisition times ranging from 1:57min to 3:01min. bSTAR datasets were then reconstructed off-line to 1mm and 1.5mm isotropic resolution using compressed sensing with a fast iterative shrinkage-thresholding algorithm (FISTA)[5]. For comparison, a product SPGR sequence with and without MT saturation preparation was used. Imaging was performed with a flip angle of 25°, TE/TR of 6/23ms, bandwidth of 110Hz/px, FOV of 240×216×144mm3, and a matrix size of 160×144×96, yielding 1.5mm isotropic resolution. Each of the MT-weighted and non-MT-weighted SPGR imaging took 2:32 min. Finally, magnetization transfer ratio (MTR) brain images were calculated pixelwise from the MT-weighted (SMT) and non-MT-weighted (SnonMT) signals using MTR = 100×(SnonMT - SMT)/SnonMT.Results
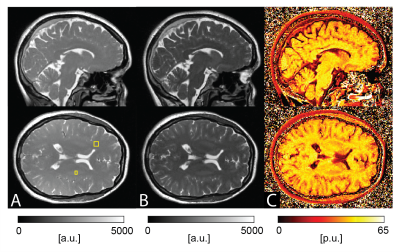
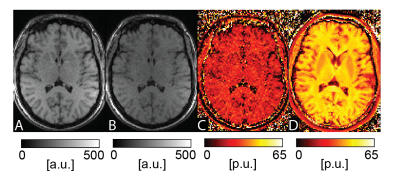
As expected, the bSSFP signal from brain tissue shows a pronounced monotonous decrease with decreasing TRF (see Figure 1) and thus MTR calculations were based on the scans with TRF of 0.14 ms (TR of 2.9ms) and 1.8 ms (TR of 4.5ms). Example bSTAR MTR images are shown in Figure 2 in both axial and sagittal orientation. At low field and within the explored upper TR boundary, bSSFP images are free of any off-resonance related image degradations and whole brain bSSFP-based MTR imaging is essentially artifact-free. The observed average MTR values of 45 p.u. in white matter and 39 p.u. in gray matter (see Figure 2) are well within the typical expectations from high-field MTR imaging. In contrast, SPGR-based MTR imaging with the product sequence was far less successful. From the overall lower scanning efficiency (due to the MT-preparation module) not only signal-to-noise ratio (SNR) is considerably reduced but also the overall achievable MT contrast is surprisingly low. For SPGR-based MTR imaging, WM MTR values of about 27 p.u. are observed which are far below typical observations at high field.Discussion
At low field off-resonances become less severe for bSSFP, thus offering excellent prospects for all bSSFP-based contrast mechanisms, such as MT. In this work, we used a half-radial dual-echo bSSFP sequence for MT-sensitized bSSFP imaging offering minimal TR and maximal readout efficiency. As a result, bSSFP imaging not only becomes essentially banding-free at 0.55T but also provides enough SNR to achieve whole-brain high-resolution MTR scanning in 4:58 min. In contrast, conventional 3D SPGR MTR imaging with 1.5mm isotropic resolution appears highly limited by SNR. The overall achievable MTR contrast with SPGR at low field, however, was not only limited by SNR but also by the overall observed MT-saturation effects. Since a product sequence with no access to the MT-preparation parameters settings was used, it remains to be investigated whether this links to a non-optimal MT preparation or the contemporary MT saturation approach with SPGR is generally limited at low field.Conclusion
At low fields, bSSFP-based MTR imaging appears to considerably outperform conventional SPGR-based approaches offering similar sensitivity and resolution for similar scan times as compared to high field MRI.Acknowledgements
This work was supported by the Swiss National Science Foundation (SNF grant No. 325230_182008)References
1. Sled JG. Modelling and interpretation of magnetization transfer imaging in the brain. NeuroImage 2018;182:128–135 doi: 10.1016/j.neuroimage.2017.11.065.
2. Horsfield MA. Magnetization Transfer Imaging in Multiple Sclerosis. J. Neuroimaging 2005;15:58S-67S doi: 10.1177/1051228405282242.
3. Bieri O, Scheffler K. Optimized balanced steady-state free precession magnetization transfer imaging. Magn. Reson. Med. 2007;58:511–518 doi: 10.1002/mrm.21326.
4. Bauman G, Bieri O. Balanced steady-state free precession thoracic imaging with half-radial dual-echo readout on smoothly interleaved archimedean spirals. Magn. Reson. Med. 2020;84:237–246 doi: 10.1002/mrm.28119.
5. Beck A, Teboulle M. A Fast Iterative Shrinkage-Thresholding Algorithm for Linear Inverse Problems. SIAM J. Imaging Sci. 2009;2:183–202 doi: 10.1137/080716542.
Figures