2706
Acquisition optimization for cortical ihMTsat imaging1McConnell Brain Imaging Centre, McGill University, Montreal, QC, Canada, 2Neurology and Neurosurgery, McGill University, Montreal, QC, Canada, 3Hotchkiss Brain Institute and Departments of Radiology and Clinical Neuroscience, University of Calgary, Calgary, AB, Canada, 4Biomedical Engineering, McGill University, Montreal, QC, Canada
Synopsis
Sequence simulations are used to compare different conventional and boosted saturation schemes and imaging protocols for the purpose of high-resolution ihMTsat cortical imaging. The simulations are constrained to a maximum of four minutes per volume. We find an optimal region for conventional saturation that produces the greatest ihMTsat. For the boosted approach, larger ihMTsat values are found with respect to the conventional approach, and ihMTsat increases with longer TRs. However, the practical utility of the boosted approach for cortical imaging is hampered by a wider PSF associated with higher turbo factors. Simulated ihMTsat findings are supported by in vivo data.
Introduction
Inhomogeneous magnetization transfer (ihMT) is an emerging brain MR contrast1 that has been demonstrated to have high specificity to myelin2. The ihMT contrast originates from the differential saturation of the dipolar pool from single (+Δ or -Δ) vs dual (±Δ) frequency saturation pulses3,4. Recently, the feasibility of ihMT imaging of the human cerebral cortex has been demonstrated5, making ihMT a promising method to specifically probe the complex cortical myeloarchitecture in vivo.A strong ihMT signal can be generated using high B1rms saturation pulses1, but there is currently no consensus on the optimal saturation parameters. It has been demonstrated that using a low-duty cycle, “boosted” saturation approach2,6 can generate elevated ihMT compared to the conventional approach (see Figure 1). This approach produces increased ihMT but requires longer TRs. This necessitates large turbo factors to generate images with sufficient nominal resolution for cortical imaging with reasonable scan times. However, the change in magnetization during the turbo-readout can impact the actual resolution achieved by these sequences, offering a trade-off between resolution and contrast.
Optimization of the ihMT acquisition is difficult due to the large number of adjustable sequence parameters. This study combines sequence simulations and in vivo data to compare ihMTsat values from conventional and boosted saturation schemes, and to visualize the impact on ihMTsat in the cortex.
Methods
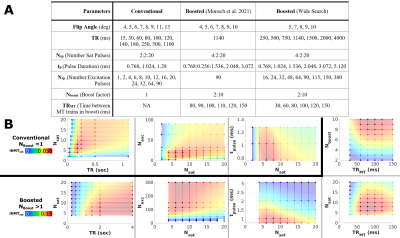
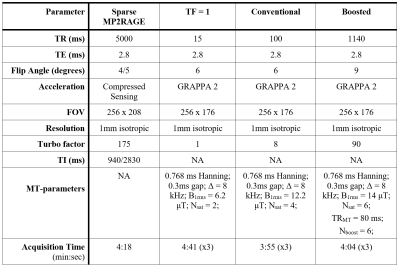
Simulations: Bloch simulations were performed as described previously7. The following tissue parameters were used for the cortex at 3T: M0A = 1, M0B = 0.075, T2B = 10 μs, T2A = 60 ms, R1,obs = 0.85 s-1, R1B = 1 s-1, R = 26 s-1, T1D = 3 ms, and a Super-Lorentzian lineshape. To constrain the search space, we set tgap to 0.3 ms, the Δ of the saturation pulses was fixed to 8 kHz, and a maximum scan time was set to 4 min/volume. The range of parameters used in the simulations are listed in Figure 2A. The B1rms of the saturation pulses was scaled to not exceed 3 W/kg, with an additional limit of 14 µT for B1rms. The steady state signal was taken from the first echo. MTsat values were generated using the simulated signal, M0A and R1,obs as inputs to a lookup table generated with Bloch simulations8. The ihMTsat value was calculated by taking the difference between the ±Δ MTsat value and the +Δ MTsat value. A PSF analysis was performed using the simulated ihMTsat signal over the turbo-readout.Imaging: MR images were collected in two healthy adults (1 female, 34-35 years old) using a 3T-PrismaFit scanner (Siemens, Germany) with a 32-channel receive coil. A custom sequence was used with radial fan beam encoding9, elliptical sampling, and customizable saturation parameters. Imaging parameters are listed in Figure 3. A B1+ map was acquired using a fast turboFlash sequence10 with 3 mm isotropic voxels and 40 sec duration. B1+ maps were upsampled to 1 mm and smoothed with a 5 mm gaussian kernel. All volumes were registered to a T1W FLASH image (TR = 14 ms, flip angle = 20 deg), which was used as input for the FreeSurfer (v7.2) recon-all pipeline, with manual edits to remove dura mater. MTsat maps were calculated as in the simulations, using a sparsely encoded MP2RAGE11 acquisition to generate R1,obs and apparent M0 maps. ΔB1+ correction of MTsat maps was performed using a model-based framework7. Finally, ihMTsat maps were generated by taking the difference between the ±Δ MTsat map and the average of the +Δ and -Δ MTsat map.
Results
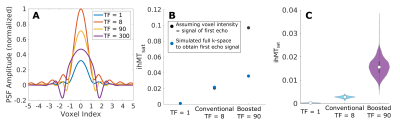
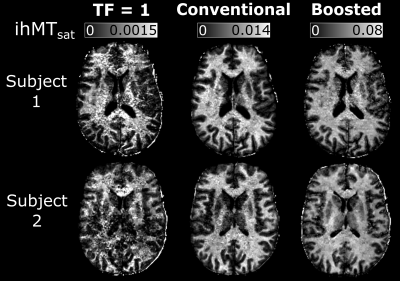
For the conventional approach, the results in Figure 2B suggest an optimal TR of 100 ms, Nexc = 6-25 and Nsat = 4-10. In comparison, the boosted approach showed a peak boost benefit with TRMT = 80 ms, and Nsat = 6-8. ihMTsat values rose with increasing Nboost, leading to long TRs and large turbo factors given the maximum scan time.The PSF analysis in Figure 4A suggests that the conventional protocol provides the best resolution for the restrictions that we imposed on the sequence parameters. For in vivo calculation of MTsat, we assume the voxel intensity is equivalent to the signal of the first echo in a centric-encoded readout. However, the image intensity is weighted by the entire turbo-readout. Figure 4B highlights the impact of the magnetization change over long turbo-readouts on ihMTsat. As expected, this assumption causes in vivo maps to have artificially increased saturation values with increasing turbo factors. However, the ihMTsat SNR will ultimately be determined by the difference between the input ±Δ and +Δ and -Δ signals.
Discussion and Conclusions
Simulation results for our imposed matrix size and time constraints showed an optimal region for high resolution, conventional saturation ihMT imaging. Boosted approaches result in increased ihMTsat but come at the cost of decreased actual resolution of the image stemming from the increased the turbo factor. Since ihMT is a low SNR measure, the ideal SNR ihMT protocol will come by finding an optimal combination of a large saturation difference between ±Δ and +Δ and -Δ signals, while not having so much signal saturation that it impacts the SNR of the input images.Acknowledgements
This project has been made possible by the Brain Canada Foundation, through the Canada Brain Research Fund, with the financial support of Health Canada and the Natural Sciences and Engineering Research Council of Canada, the Fonds de recherche du Québec – Santé, Healthy Brains for Healthy Lives, the Campus Alberta Innovates Program, Quebec BioImaging Network, Jeanne Timmins Costello and Dr. David T.W. Lin.
References
1. Varma G, Duhamel G, De Bazelaire C, Alsop DC. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med. 2015;73(2):614-622. doi:10.1002/mrm.25174
2. Duhamel G, Prevost VH, Cayre M, et al. Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. Neuroimage. 2019;199:289-303. doi:10.1016/j.neuroimage.2019.05.061
3. Lee JS, Khitrin AK, Regatte RR, Jerschow A. Uniform saturation of a strongly coupled spin system by two-frequency irradiation. J Chem Phys. 2011;134(23):1-6. doi:10.1063/1.3600758
4. Swanson SD, Malyarenko DI, Fabiilli ML, Welsh RC, Nielsen JF, Srinivasan A. Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magn Reson Med. 2017;77(3):1318-1328. doi:10.1002/mrm.26210
5. Munsch F, Varma G, Taso M, et al. Characterization of the cortical myeloarchitecture with inhomogeneous Magnetization Transfer imaging (ihMT). Neuroimage. 2021;225(October 2020):117442. doi:10.1016/j.neuroimage.2020.117442
6. Varma G, Girard OM, Mchinda S, et al. Low duty-cycle pulsed irradiation reduces magnetization transfer and increases the inhomogeneous magnetization transfer effect. J Magn Reson. 2018;296:60-71. doi:10.1016/j.jmr.2018.08.004
7. Rowley CD, Campbell JSW, Wu Z, et al. A Model-based Framework for Correcting B1+ Inhomogeneity Effects in Magnetization Transfer Saturation and Inhomogeneous Magnetization Transfer Saturation Maps. Magn Reson Med. 2021;86(4):2192-2207. doi:10.1002/mrm.28831
8. Rowley CD, Wu Z, Leppert IR, et al. Inhomogeneous magnetization transfer saturation (ihMT): efficient centric-encoded GRE implementation with B1 inhomogeneity correction. In: Proceedings of the ISMRM & SMRT Virtual Conference & Exhibition. ; 2020:3136.
9. Saranathan M, Glockner J. Three-dimensional dixon fat-water separated rapid breathheld imaging of myocardial infarction. J Magn Reson Imaging. 2013;38(6):1362-1368. doi:10.1002/jmri.24113
10. Chung S, Kim D, Breton E, Axel L. Rapid B1+mapping using a preconditioning RF pulse with turboFLASH readout. Magn Reson Med. 2010;64(2):439-446. doi:10.1002/mrm.22423
11. Mussard E, Hilbert T, Forman C, Meuli R, Thiran JP, Kober T. Accelerated MP2RAGE imaging using Cartesian phyllotaxis readout and compressed sensing reconstruction. Magn Reson Med. 2020;84(4):1881-1894. doi:10.1002/mrm.28244
12. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Reson Med. 2008;60(6):1396-1407. doi:10.1002/mrm.21732
Figures
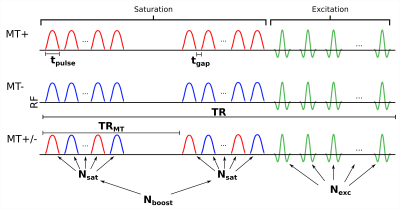
Figure 1: A custom MT-weighted FLASH sequence diagram highlighting the parameters that can be modified to optimize contrast. The conventional saturation approach uses a single train of Nsat saturation pulses followed by Nexc excitation pulses. In the boosted approach, Nboost trains of Nsat pulses are applied at a time interval TRMT, prior to the excitation pulses. For this study, the following parameters were modified in sequence simulations: tpulse, TRMT, Nsat, Nboost, Nexc, TR.