2705
Understanding inhomogeneous MT (ihMT) in multi-parameter mapping of human brain: Towards larger ihMT, higher resolution, and influence of T1d1Clinical Sciences, Medical Radiation Physics, Lund University, Lund, Sweden, 2Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3NMR Methods & Development Group, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 4Felix Bloch Institute for Solid State Physics, Leipzig University, Leipzig, Germany
Synopsis
Inhomogenous Magnetization Transfer (ihMT), the differential response to irradiation at single and dual frequency offsets, is more complex than MT approaches. Expressing ihMT in terms of MT-saturation (ihMTsat) is a first step to quantification as it corrects for underlying T1 and B1+. Larger ihMTsat was observed for smaller frequency offsets, which is explained using MTsat as proxy for bound pool saturation. For longer TR, ihMTsat increased faster than MTsat indicating recovery of dipolar order as ihMTsat increased super-linearly to 1.1pu in WM and 0.2pu in GM at TR=52ms. ihMTsat mapping in vivo was performed at 1.3mm isotropic resolution.
Introduction
Magnetization Transfer (MT) is induced by saturation of “bound “magnetization of macromolecules by off-resonance RF. Interaction with dipolar spin order D can reduce net saturation, which has been dubbed inhomogeneous MT (ihMT) and is observed as differential response to irradiation at single and dual frequency offsets (±Δ)1. ihMT in brain is promising because of its presumed sensitivity to myelin2 but its measurement offers more degrees-of-freedom than MT.We implemented ihMT into multi-parameter mapping (MPM) at 3T to calculate MT-saturation (MTsat) and its dipolar analog (ihMTsat),3 which describe the bound pool saturation as indirectly observed by free water, corrected for underlying variations in flip angle, B1 and T1 relaxation4. Sequence parameters were varied to maximize ihMTsat and achieve an isotropic resolution of 1.3mm. ihMT measurements can be interpreted by using MTsat as proxy for the saturation of the bound pool, which is not directly observable.
Theory
The coupling to dipolar spin order D occurs solely within the bound pool M0b (characterized by lineshape gb(2πΔ) of second moment ωd) (Fig. 1). RF irradiation with ω1=γB1 at a single Δ saturates the bound pool:[1] dMzb/dt = –πω12 gb(2πΔ) [Mzb – 2π|Δ|D/ωd]
The dipolar “damping” disappears when RF is irradiated at positive and negative Δ simultaneously, mimicked by trains of MT-pulses with alternating offsets. The fractional reduction of Mzb is transferred to free water and observed as MTsat (Fig. 1). Obviously, the proportion of Mzb and D strongly influences ihMT. Though D is small compared to M0b, its relaxation time T1d is in the millisecond range, whereas the exchanging pools can be strongly saturated due to T1≈1s. The MT-FLASH signal is approximated by4
[2] S±± = S0 α R1TR / (R1TR + α2/2 + δ±±) S++ and S-- denote positive and negative offsets and S+– and S–+ alternating signs with corresponding MT-saturations (δ++/δ--/δ+–/δ–+ in percent units [p.u.]) . δ+– and δ–+ are larger than δ++ and δ--. The latter differ slightly due to shifted gb(2πΔ) . This is eliminated by averaging:
[3a] MTsatdual = ( δ+–+ δ–+)/2
[3b] MTsatmono = ( δ++ + δ--)/2
ihMTsat is defined as their difference3:
[4] ihMTsat = MTsatdual – MTsatmono
Methods
Informed and consenting healthy adults were scanned at 3T (Siemens SkyraFit) using a 32-channel receive coil. In an MT-FLASH sequence for MPM, the Gaussian MT-pulse was replaced with 8 Gaussian pulses of 1ms duration and 0.12ms spacing allowing to alternate the sign of Δ to mimic dual frequency irradiation.To study the impact of sequence parameter variation, sagittal 3D volumes were acquired at 2mm resolution, 128x112x80 matrix, 7/8 partial-Fourier in phase and slice. After 10° readout, six bipolar gradient echoes with 500Hz/pixel readout bandwidth were acquired at TE=2.3/4.54/6.78/9.02/11.26/13.5ms.
Parameters were obtained using the hMRI toolbox (hMRI.info)5. FSL was used for registration to a T1-weighted reference, brain extraction, and whole-brain histogram analysis of MTsat and ihMTsat maps.
Experiment 1: To study the transfer of saturation from the bound pool, TR was increased through 28,34,40,46 to 52ms. The MT pulses were applied with 63° at the previously recommended offset of ±8kHz6.
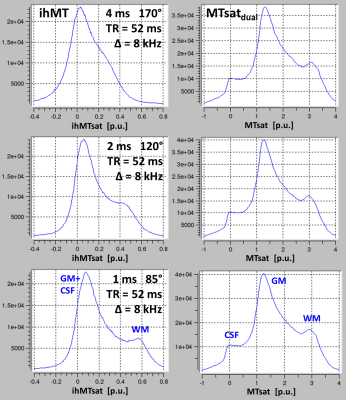
Experiment 2: The MT pulse duration was varied through 4, 2, 1ms at TR=52ms offset to study effects of T1d on ihMTsat.
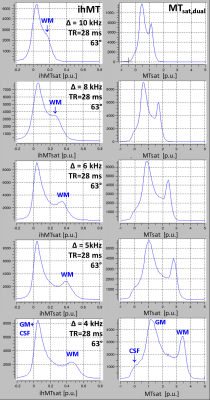
Experiment 3: To study the dependence of ihMTsat on frequency offset, Δ was varied across ±4kHz, ±5kHz, ±6kHz, ±8kHz, and ±11kHz at TR=28ms with MT-pulses of 63°.
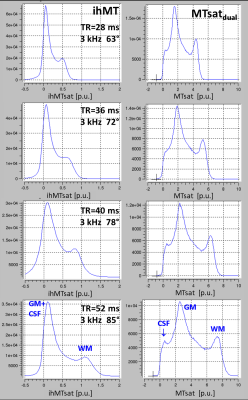
Experiment 4: Experiment 1 was repeated with 3kHz offset and TR =27.8, 36, 46, 52ms; all at highest SAR level (99%) by adjusting the flip angle of the MT-pulse (63°,70°,72°,85°).
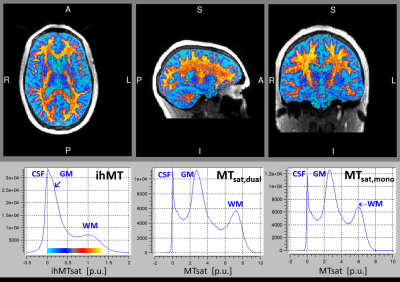
The final protocol featured TR=52ms with GRAPPA in phase and slice direction, and 24°, 6° and 6° readout for T1-, PD- and ihMT-preparation by MT-pulses of 85° at ±3kHz – the lowest offset without “direct” saturation. SAR levels were between 97% and 100% in different subjects. Acquisition at 1.3mm isotropic resolution took 2x2:21+4x6:08=29 minutes.
Results and Discussion
Experiment 1(no figure): MTsatdual and MTsatmono increased roughly linearly with TR indicating transfer of saturation to free water. ihMTsat increased more strongly than MTsat, indicating restoration of D during TR.Experiment 2/Figure 2: ihMTsat decreased strongly with pulse duration, but MTsatdual was largely unaffected. Assuming restoration of D during free relaxation intervals between the MT-pulse trains, the observed ihMTsat values were consistent with experiment 1, as free relaxation intervals for TR=28/36/52 ms corresponded to those for 4/2/1 ms pulse duration.
Experiment 3/Figure 3: ihMTsat increased when decreasing Δ, without exhibiting a maximum at about 8kHz as reported for CW saturation. The concomitant increase in MTsatdual indicated increasing saturation of Mzb.
Experiment 4/Figure 4: At 3kHz offset and ~100% SAR, ihMT increased super-linearly with TR, due to restoring D but keeping Mzb saturated This suggests that T1d may be considerably longer than 15ms in brain, motivating the use of interleaved trains of repeated MT and readout pulses for ihMT measurements7.
Figure 5: The ihMTsat map at 1.3mm resolution was of reasonable SNR with values of 0.2/1.1 pu in GM/WM emphasizing ihMT in subcortical WM.
Conclusion
The simple structure of MT-FLASH allows for an intuitive understanding for optimizing ihMT. Estimating the physical properties of the dipolar reservoir will require quantitative modeling of the exchanging Zeeman pools.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n° 616905. This project has received funding from the European Union's Horizon 2020 research and innovation programme under the grant agreement No 681094, and is supported by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137. This project has received funding from the BMBF (01EW1711A & B) in the framework of ERA-NET NEURON.
The Max Planck Institute for Human Cognitive and Brain Sciences has an institutional research agreement with Siemens Healthcare. NW was a speaker at an event organized by Siemens Healthcare and was reimbursed for the travel expenses.
References
1. Varma G, Girard OM, Prevost VH, Grant AK, Duhamel G, Alsop DC. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J Magn Reson. 2015 260:67-76.
2. Duhamel G, Prevost VH, Cayre M, Hertanu A, Mchinda S, Carvalho VN, Varma G, Durbec P, Alsop DC, Girard OM: Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. NeuroImage 199 (2019) 289–303.
3. Helms G, Vaculčiaková L, Pine KJ, Möller HE, Weiskopf N. Measuring inhomogeneous MT (ihMT) in human brain by multi-parameter mapping (MPM). 27. Virtual Meeting of the ISMRM. Proc ISMRM 2021 27:1840.
4. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetisation transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Res Med. 2008;60:1396–1407.
5. Tabelow K, Balteau E, Ashburner J, Callaghan MF, Draganski B, Helms G, Kherif F, Leutritz T, Lutti A, Phillips C, Reimer E, Ruthotto L, Seif M, Weiskopf N, Ziegler G, Mohammadi S. hMRI - A toolbox for quantitative MRI in neuroscience and clinical research. Neuroimage. 2019;194:191-210.
6. Mchinda S, Varma G, Prevost VH, Le Troter A, Rapacchi S, Guye M, Pelletier J, Ranjeva J-P, Alsop DC, Duhamel G, Girard OM. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn Reson Med. (2017) 79:2607-2619.
7. Varma G, Munsch F, Burns F, Duhamel G, Girard OM, Guidon A, Lebel RM, Alsop DC. Three‐dimensional inhomogeneous magnetization transfer with rapid gradient‐echo (3D ihMTRAGE) imaging. Magn Reson Med. 2020 84:2964-298.
Figures
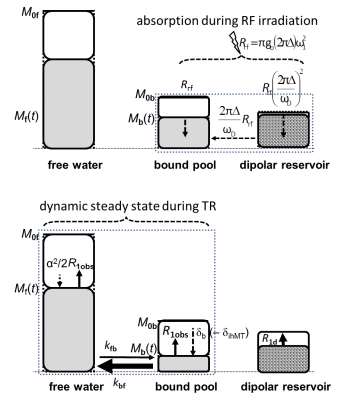
Top: Bound pool and dipolar reservoir during off-resonance RF irradiation. The dipolar reservoir has been largely restored by R1d, while Mzb is in a dynamic steady state of exchange with free water. Only at a single offset Δ, transfer of dipolar order (dashed arrow) reduces the saturation of Mzb (Eq. [1]).
Bottom:
The bound pool starts
strongly saturated after RF absorption, while readout saturates free water by 1–cosα ≈ α2/2. The dynamic
steady state during TR is governed by the longitudinal relaxation rate R1obs and the transfer of fractional saturation δb (–δihMT) to free water.