2692
Visualising and interacting with diffusion MRI microstructural models using FSL-DIVE1University of Oxford, OXFORD, United Kingdom, 2Mathematics, University of Oxford, Oxford, United Kingdom
Synopsis
We introduce FSL-DIVE, an interactive visualisation software for diffusion microstructure modelling. This software allows the user to (i) flexibly set up a multi-compartment microstructure model, including fibre orientation modelling and restricted diffusion, (ii) choose different acquisition parameters, (iii) change the model parameters and analyse the model behaviour through multiple bespoke plots, (iv) save the predicted data (including noise) for any specified acquisition scheme. We hope this tool may be useful for teaching diffusion microstructure modelling, for developing new models, or for getting a better understanding of existing models.
Introduction
Microstructure modelling is an exciting area of diffusion MR imaging. Biophysically inspired models are used to infer tissue microstructure correlates of diffusion MR signals, with the aim to provide specific and quantitative markers of tissue state. Many such models have been proposed to estimate brain tissue features such as soma size, axon diameter or fibre orientation and dispersion [1]. These microstructural models are typically composed of different compartments, each with specific geometric and diffusion properties, leading to a signature diffusion profile [2]. While fitting these complex models to diffusion data can hopefully yield more specific, interpretable quantities (e.g., axon diameter or density) than phenomenological models (e.g., tensor FA), it is also important to realise the limitations that these models can have. For example, they are a simplified version of reality, and they can be over-parameterised, which is problematic for parameter fitting [3]. In addition, it is important to understand the different experimental conditions under which these models can be useful. To help get a handle on diffusion microstructure models, we developed an open-source visualisation tool that allows the user to flexibly specify a multi-compartment diffusion model, change the model parameters, immediately observe their effect on the predicted signals, and flexibly alter the diffusion acquisition protocol. We hope that this tool will be useful for probing diffusion models, for developing new models, and for teaching.Overview of the software
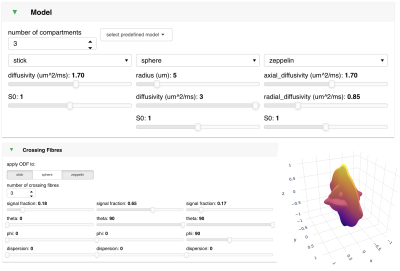
The software is web-based and can be run locally or hosted on a remote web server. The user interacts with the software via the web-browser. It is written in Python and is built upon the Bokeh [4] and Panel [5] libraries for interactive visualisation. The app comprises three components: (a) model specification, (b) acquisition, and (c) visualisation. Figures 1-4 show some of the panels including an example model (SANDI [6]) visualised in various ways.a. Model specification (Figure 1): the user can specify any number of compartments which are added together to make up the overall signal prediction. In addition to compartments with free or hindered Gaussian diffusion (stick, ball, zeppelin, tensor), we also allow two types of restricted compartments (sphere, to model cell bodies, and cylinder, to model axons), and a compartment for stationary water (dot). The user can also apply an orientation distribution function to all or a subset of the compartments. These are modelled as an arbitrary number of crossing Watson distributions (including stick-like as a special case).
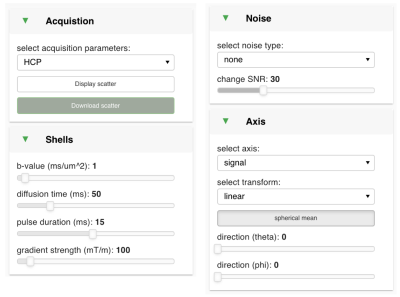
b. Acquisition (Figure 4): currently, the interface assumes a Stejskal-Tanner acquisition [7]. The user can specify a shell (in terms of b-value or its components, i.e., diffusion time, gradient amplitude, and duration). The user can also load a bespoke acquisition protocol and visualise the predicted data under said protocol. Noise can be added to the predictions and the data saved for further analysis (e.g., fitting).
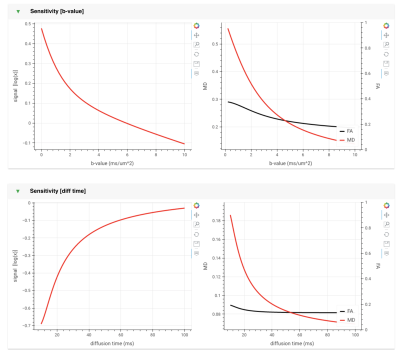
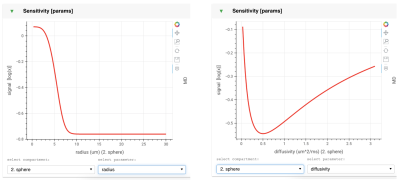
c. Visualisation: multiple panels allow the user to study the model sensitivity to b-value, diffusion time, and gradient orientations (figure 2). There is also a panel for looking at sensitivity to individual model parameters (figure 3). Together, these panels allow a deeper understanding of the model behaviour and limitations.
Future work
There are two immediate improvements to the current framework which are underway, one in the modelling and one in the acquisition. First, we will extend the compartment modelling to allow for exchange between compartments. Second, we will include a more flexible definition of the diffusion acquisition, for example expanding to tensor encoding schemes [8].Software
https://git.fmrib.ox.ac.uk/fsl/DIVESee also demo video: https://www.youtube.com/watch?v=9c2-Y1DtbVQ
Acknowledgements
This work is supported by the Wellcome Trust (215573/Z/19/Z and 221933/Z/20/Z). We would like to thank Paul McCarthy (FSLeyes) for his input.References
[1] F. Sepehrband, D. C. Alexander, N. D. Kurniawan, D. C. Reutens, and Z. Yang, ‘Towards higher sensitivity and stability of axon diameter estimation with diffusion-weighted MRI’, NMR in Biomedicine, vol. 29, no. 3, pp. 293–308, 2016, doi: 10.1002/nbm.3462.
[2] D. S. Novikov, V. G. Kiselev, and S. N. Jespersen, ‘On modeling’, Magnetic Resonance in Medicine, vol. 79, no. 6, pp. 3172–3193, 2018, doi: 10.1002/mrm.27101.
[3] D. S. Novikov, E. Fieremans, S. N. Jespersen, and V. G. Kiselev, ‘Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation’, NMR in Biomedicine, vol. 32, no. 4, p. e3998, 2019, doi: 10.1002/nbm.3998.
[4] Bokeh Development Team, Bokeh: Python library for interactive visualization. 2018. [Online]. Available: https://bokeh.pydata.org/en/latest/
[5] P. Rudiger et al., holoviz/holoviews: Version 1.13.3. Zenodo, 2020. doi: 10.5281/zenodo.3904606.
[6] M. Palombo et al., ‘SANDI: A compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI’, NeuroImage, vol. 215, p. 116835, Jul. 2020, doi: 10.1016/j.neuroimage.2020.116835.
[7] E. O. Stejskal and J. E. Tanner, ‘Spin Diffusion Measurements: Spin Echoes in the Presence of a Time‐Dependent Field Gradient’, J. Chem. Phys., vol. 42, no. 1, pp. 288–292, Jan. 1965, doi: 10.1063/1.1695690.
[8] F. Szczepankiewicz, J. Sjölund, F. Ståhlberg, J. Lätt, and M. Nilsson, ‘Tensor-valued diffusion encoding for diffusional variance decomposition (DIVIDE): Technical feasibility in clinical MRI systems’, PLOS ONE, vol. 14, no. 3, p. e0214238, Mar. 2019, doi: 10.1371/journal.pone.0214238.
Figures