2685
Acquisition and finite element analysis of 4DFlow-MR functional angiography in brain and neck1MRI Department, Fundación Argentina para el Desarrollo en Salud, Mendoza, Argentina, 2MRI Department, Fundación Escuela de Medicina Nuclear, Mendoza, Argentina, 3FI, Universidad de Mendoza, Mendoza, Argentina, 4FGQNYCS, Comisión Nacional de Energía Atómica, Buenos Aires, Argentina, 5Instituto Balseiro, Universidad Nacional de Cuyo, San Carlos de Bariloche, Argentina, 6FCEN, Universidad Nacional de Cuyo, Mendoza, Argentina, 7MRI Department, Fundación Argentina para el Desarrollo en Salud, Mendoza, Argentina, 8Hemodynamics, Fundación Argentina para el Desarrollo en Salud, Mendoza, Argentina, 9Research Department, Philips Healthcare, Barueri/San Paulo, Brazil
Synopsis
The abscence of an angiographic functional analysis of the head and neck regions opens the possibility of applying the 4D Flow technique to quantify hemodynamics and blood flow parameters. Through a Python algorithm using Finite Elements Method the dynamic blood behavior was analyzed, obtaining a complete correlation with the clinical diagnosis.
Indroduction
4D Flow is a hemodynamic non-invasive MR technique which has been widely used for evaluating cardiac pathologies and large-caliber vessels [1]. This sequence allows the acquisition of flow data encompassing vascular structures and permits to measure and visualize their temporal evolution. Unlike the arteries involved in the cardiovascular system (25-35 mm of diameter), the carotid and cerebral arteries have calibers between 3 and 9 mm and a lower hemodynamic response function (HRF) amplitude due to their dimensions and anatomical position. In order to model the hemodynamic on the artery, finite element method (FEM) is a numerical method used to analyze blood flow simulating the geometry of the system and considering real flow conditions such as pulsatile motions [2,3].The codification of the blood flow is performed with a 3D phase contrast technique based on linearly independent bipolar gradients, by which the temporal codification of the velocity in the three directions of space is achieved. The phase shift ($$$\phi$$$) of the spins change when the magnetization is subjected to an encoding gradient $$$G(t)$$$ applied for a short period of time $$$\delta$$$ following a $$$\pi/2$$$ RF pulse. In this sequence, the gradient is applied right before and after the $pi$ refocuse pulse; the first one produces a phase change and the second cancels the $$$\phi$$$ of the static spins. On the other hand, non-stationary spins will have a $$$\phi$$$ related with the following equation:$$\phi=\gamma G\delta\Delta tv$$where $$$\gamma$$$ is the gyromagnetic ratio, $$$Delta t$$$ is the period of time between the application of the bipolar gradients and $$$v$$$ is the proton’s speed which can be deducted with this technique.
We aim to adequate this technique to quantify and model the hydrodynamics parameters of the blood in the arteries of the brain. In order to achieve this we subdivided the geometric model into a finite number of elements to simulate complex blow flows and their hemodynamics.
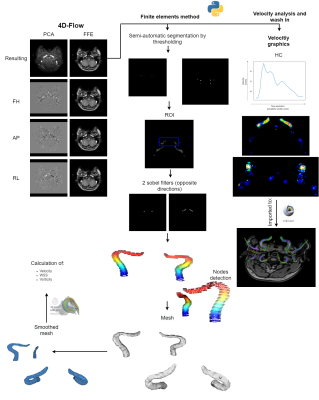
Materials and methods
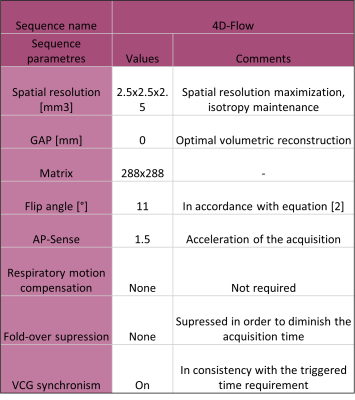
This retrospective study was approved by the Institutional Review Board. The analysis was carried out in 8 patients, from which 6 were healthy controls (HC) and 2 were pathological cases (aneurysms). The images were acquired in a 1.5 T Ingenia Philips scanner, using a HeadNeckSpine coil. The angiographic protocol includes the sequences described in Table [1]. An algorithm was developed to make an automatic thresholding in order to generate a semi-automatic segmentation, as shown in Figure [1].Nodes were defined and then a neighbouring function was used in order to calculate the cells that composed the mesh, which was afterwards smoothed with a taubin filter in order to achieve an optimal result. This routine was implemented using vtk, open3d and pyvista libraries (Python). Also, we characterized the maximum variation of velocity and presented it as a wash-in map. Additional information on the programming algorithms are shown in Figure [1].Assuming blood as a Newtonian fluid, we used FEM for analysing discrete spatial information. Through this model we could calculate from the velocity information (4D-Flow) other variables such as Wall Shear Stress, Vorticity and Energy Loss. These hemodynamic variables were calculated from the mesh file (.ply) with a FEMQ-4D software (Matlab 2019).For the HC, the information was compared with the clinical diagnosis and the other MRI information. In the patients with vascular pathologies, the post-processed data was additionally contrasted with information acquired from angiography procedures.Results and conclusions
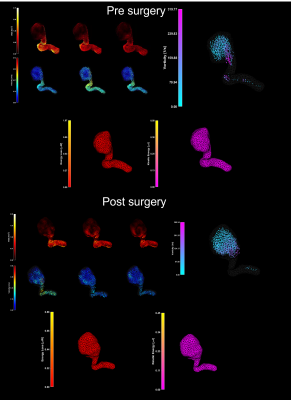
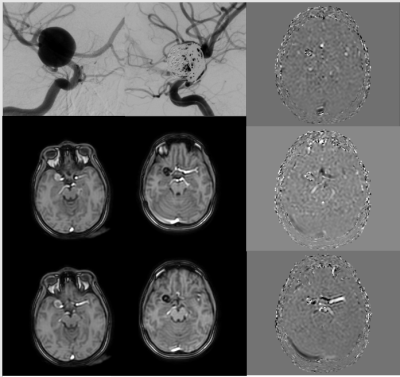
A mesh was generated through the Python routine allowing the visualization of multiple arteries by a unique semi-automatic segmentation (Figure [1]). The temporal evolution of the velocity and the hemodynamic parameters such as WSS, vortex, loss of energy, and kinetic energy were calculated. Additionally, we obtained flow values including wash-in and blood flow. In the HC, a laminar response was shown (analyzed through velocity, the wash-in and the blood flow) and there was no presence of vortex, WSS (not expected by the specialists), or kinetic energy loss. On the other hand, the pathological cases information was consistent with the clinical report and the angiographical information. A postoperative analysis was made and there were no coil artifacts that affect the quality of the image (Figure [2]).Regarding the hemodynamics parameters, the velocity values in the interior of the aneurysm after the surgery had a more homogeneous response. The vortex was decreased by 33%, wall shear stress by 62% and no vortex were measured on not expected regions. (Figure [3]).
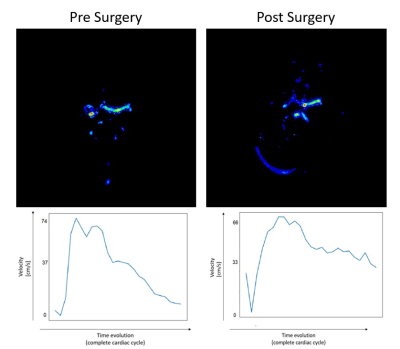
About the wash-in information, it was feasible to appreciate an increase in blood velocity at the exit of the aneurysm, as had been seen in the angiographic diagnosis(Figure [4]). An attenuation of the HRF on the pathological patient was verified and there were no changes for HC.
Discussion
Acquisition and processing routines suitable for clinical practice related to the analysis of angiographic pathology were designed, although the execution times of the sequence are similar to those used in cardiology, they are still considered long compared to other functional techniques. The advantages from the modeling of irregular geometries were maximized choosing FEM as a processing method. Despite the fact that it required big amounts of data entry and boundary conditions, a computational algorithm has been achieved according to the demands that this type of study has.Acknowledgements
This project is part of a research cooperation with Philips Healthcare. We wish to extend our special thanks to Horacio Kozaczuk.References
[1] Dyverfeldt, P., Bissell, M., Barker, A. J., Bolger, A. F., Carlhäll, C. J., Ebbers, T., ... & Markl, M. (2015). 4D flow cardiovascular magnetic resonance consensus statement. Journal of Cardiovascular Magnetic Resonance, 17(1), 1-19.
[2] Frey, Sergio & Hughes, Thomas. (1992). Stabilized finite element methods: I. Application to the advective-diffusive model. Computer Methods in Applied Mechanics and Engineering. 95. 253-276. 10.1016/0045-7825(92)90143-8.
[3]Liu, H., Lan, L., Abrigo, J., Ip, H. L., Soo, Y., Zheng, D., ... & Leng, X. (2021). Comparison of Newtonian and Non-newtonian Fluid Models in Blood Flow Simulation in Patients With Intracranial Arterial Stenosis. Frontiers in Physiology, 12.