2681
Diffusion magnetic resonance imaging in genetic and idiopathic dystonias1Cardiff University Brain Imaging Centre, Cardiff University, Cardiff, United Kingdom, 2Neuroscience and Mental Health Research Institute, Cardiff University, Cardiff, United Kingdom, 3Image Sciences Institute, University Medical Centre Utrecht, Utrecht, Netherlands
Synopsis
Dystonia is a hyperkinetic movement disorder involving repetitive or sustained muscle contractions. Its pathophysiology is poorly understood, limiting therapeutic advancement. A systematic literature review on diffusion MRI in dystonia was performed to gain insight into microstructural white matter changes that may contribute to pathology. Of 403 identified records, 40 met the criteria for inclusion. The most consistent diffusion abnormalities for both genetic and idiopathic dystonia forms were lower FA values or reduced number of tractography streamlines in regions connecting brainstem, cerebellum, basal ganglia and sensorimotor cortex, with some genotype and phenotype specific differences identified.
Introduction
Dystonia is a movement disorder involving repetitive or sustained muscle contractions, affecting up to 120/100,000 population. It causes abnormal posturing, pain and disability, potentially impacting education and employment. There are both Mendelian inherited and idiopathic forms, and single or multiple muscle groups can be affected. Pharmacological treatments have limited efficacy; with injectable neurotoxin for focal/segmental forms and Deep Brain Stimulation (DBS) for more generalised forms. Research to date has demonstrated no macroscopic neuroimaging changes in dystonia, but multimodal imaging implicates the brain motor circuitry, in particular the sensorimotor cortex, basal ganglia and cerebellum, further reinforced by microscopic morphological differences in the same regions in animal model and histopathological studies (figure 1). Here, we systematically review the diffusion MR literature in dystonia, assessing methodological limitations in addition to highlighting consistent findings which may implicate microstructural morphological changes, important in dystonia pathogenesis.Methods
Embase and PubMed databases were searched up to October 2021 for search terms relating to Diffusion MRI and dystonia, including terms for different dystonia phenotypes and known mendelian inherited genetic forms of dystonia. The search terms used, as well as inclusion and exclusion criteria, are shown in figure 2. Of 403 identified records, 40 studies met the criteria for inclusion.Results
Of the 40 studies included (figure 3), all were performed at either 1.5T (n=7)1-7 or 3T (n=33)8-40. The number of diffusion gradient directions ranged from 6 (n=5)1-5, 15-29 (n=4), 30-59 (n=15) and ≥60 (n=13), and were not reported in one study7. B-values used ranged from 700-1000s/mm2 in all but one study, which had a b-value of 1500s/mm2. No studies used multiple b-values. Only 14 of the studies used isotropic or close to isotropic voxels (slice thickness <1.1x in-plane resolution). In-plane voxel dimensions were ≤2mm in all but 3 studies3,12,20, and slice thickness ranged from <2mm (n=15), 2 to ≤5mm (n=19) and 5mm (n=4)1,2,4,5. Preprocessing was generally limited to motion and eddy current correction, with 4 additionally undertaking susceptibility distortion correction. All studies used DTI (diffusion tensor imaging), with only two stating the estimation strategy (both non-linear estimation)17,18; most other studies used software that by default employ an ordinary linear least squares approach.DTI analysis methods involved a targeted region-based approach (n=14), whole brain/white matter approach (n=11) or a combination (n=15, with n=5 using whole brain approach to define ROIs1-3,11,22). These studies analysed DTI measures within an ROI (n= 15), performed tractography (n=18), undertook voxel-by-voxel based analysis (n=11) or performed TBSS (n=13). One study additionally assessed local diffusion homogeneity27, and two used a graph theory approach29,34. Multiple comparison correction involved Bonferroni correction (n=8), false discovery rate correction (n=7), family wise error rate correction (n=8) and correction at the cluster level (with a cluster extent threshold and p<0.025-0.001 n=8, threshold free cluster enhancement n=5). In 9 studies the approach was either not stated (n=1)13 or there was no correction undertaken (n=8)4,5,17,22,23,26,27,36. Cohort sizes varied from ≤10 (n=8)8,10,12,17,20,35,36,39, 10-20 (n=14)1,2,4,6,13-16,18,23,26,30,31,38, 20-30 (n=7)3,7,11,21,27,33,40 and >30 (n=11)5,9,19,22,24,25,28,29,32,34,37. All groups were compared to an age and gender matched control cohort, one which used a disease control rather than healthy control cohort.
Across the genetically homogenous cohorts, key findings included lower FA values and number of tractography streamlines in sensorimotor cortex subgyral white matter, cerebellar outflow tracts, brainstem and tracts between basal ganglia and cortex2,8,10,15,35,36, with some intermediate changes in non-manifesting gene carriers (THAP1 mutations)1,31. Amongst idiopathic cohorts, lower FA values and tractography streamline counts were also observed, most commonly in basal ganglia-cortical pathways and cerebellar regions5-7,12,16,18-20,23-26,37,40; although a number of studies reported higher FA values in regions including the internal capsule, thalamic regions, brainstem and supplementary motor cortex3,4,7,21,24,25,38. Mixed genetic and idiopathic studies (n=6)9,11,30,32-34 have indicated genotype specific differences with lower tractography streamline counts in genetic dystonias, and phenotypic specific differences related to the sensorimotor subcortical white matter.
Discussion
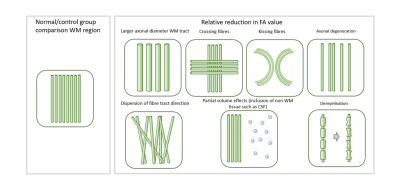
Overall, diffusion imaging findings across genetic and idiopathic forms of dystonia consistently include microstructural changes in pathways between the cerebellum, brainstem, basal ganglia and sensorimotor cortex regions (figure 4), although these represent biologically non-specific measures (figure 5). We identified substantial methodological variability across the studies, with many using large voxels, increasing potential for partial volume effects. A number of potential artefact sources were not accounted for, such as signal drift, Gibbs ringing, Rician noise bias, thermal noise, gradient deviations, b1 bias, Nyquist ghosts and outliers. The ordinary linear least squares approach to diffusion tensor estimation is a routinely used approach, although weighted linear least square or non-linear approaches, especially with a noise estimate included, can improve tensor estimation accuracy and precision. Some studies undertook no multiple comparison correction, heightening the risk of false positive results. Cohort sizes were often fairly limited, and whilst well matched for age and gender, there was little matching for non-motor symptoms such as anxiety and OCD traits which are associated with dystonic disorders.Conclusion
The white matter pathways involved in the motor networks show abnormalities in genetic and idiopathic dystonias compared to controls, with some evidence of genotypic and phenotypic specific differences. Our review demonstrates that there is substantial scope for use of more advanced imaging approaches in future studies.Acknowledgements
No acknowledgement found.References
[1] Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol. 2004;56(2):283-6.
[2] Carbon M, Kingsley PB, Tang C, Bressman S, Eidelberg D. Microstructural white matter changes in primary torsion dystonia. Movement disorders : official journal of the Movement Disorder Society. 2008;23(2):234-9.
[3] Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer's cramp. Arch Neurol. 2009;66(4):502-8.
[4] Colosimo C, Pantano P, Calistri V, Totaro P, Fabbrini G, Berardelli A. Diffusion tensor imaging in primary cervical dystonia. Journal of neurology, neurosurgery, and psychiatry. 2005;76(11):1591-3.
[5] Fabbrini G, Pantano P, Totaro P, Calistri V, Colosimo C, Carmellini M, et al. Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. Eur J Neurol. 2008;15(2):185-9.
[6] Kostic VS, Agosta F, Sarro L, Tomic A, Kresojevic N, Galantucci S, et al. Brain structural changes in spasmodic dysphonia: A multimodal magnetic resonance imaging study. Parkinsonism & related disorders. 2016;25:78-84.
[7] Prell T, Peschel T, Kohler B, Bokemeyer MH, Dengler R, Gunther A, et al. Structural brain abnormalities in cervical dystonia. BMC neuroscience. 2013;14:123.
[8] Bruggemann N, Heldmann M, Klein C, Domingo A, Rasche D, Tronnier V, et al. Neuroanatomical changes extend beyond striatal atrophy in X-linked dystonia parkinsonism. Parkinsonism & related disorders. 2016;31:91-7.
[9] Bianchi S, Battistella G, Huddleston H, Scharf R, Fleysher L, Rumbach AF, et al. Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Movement disorders : official journal of the Movement Disorder Society. 2017;32(4):560-8.
[10] Jochim A, Li Y, Zech M, Lam D, Gross N, Koch K, et al. Microstructural white matter abnormalities in patients with COL6A3 mutations (DYT27 dystonia). Parkinsonism & related disorders. 2018;46:74-8.
[11] Li HF, Yang L, Yin D, Chen WJ, Liu GL, Ni W, et al. Associations between neuroanatomical abnormality and motor symptoms in paroxysmal kinesigenic dyskinesia. Parkinsonism & related disorders. 2019;62:134-40.
[12] Bonilha L, de Vries PM, Hurd MW, Rorden C, Morgan PS, Besenski N, et al. Disrupted thalamic prefrontal pathways in patients with idiopathic dystonia. Parkinsonism & related disorders. 2009;15(1):64-7.
[13] Battistella G, Kumar V, Simonyan K. Connectivity profiles of the insular network for speech control in healthy individuals and patients with spasmodic dysphonia. Brain structure & function. 2018;223(5):2489-98.
[14] Mantel T, Altenmuller E, Li Y, Lee A, Meindl T, Jochim A, et al. Structure-function abnormalities in cortical sensory projections in embouchure dystonia. NeuroImage Clinical. 2020;28:102410.
[15] Blood AJ, Waugh JL, Munte TF, Heldmann M, Domingo A, Klein C, et al. Increased insula-putamen connectivity in X-linked dystonia-parkinsonism. NeuroImage Clinical. 2018;17:835-46.
[16] Blood AJ, Kuster JK, Woodman SC, Kirlic N, Makhlouf ML, Multhaupt-Buell TJ, et al. Evidence for altered basal ganglia-brainstem connections in cervical dystonia. PLoS One. 2012;7(2):e31654.
[17] Merchant SHI, Frangos E, Parker J, Bradson M, Wu T, Vial-Undurraga F, et al. The role of the inferior parietal lobule in writer's cramp. Brain. 2020.
[18] Berndt M, Li Y, Gora-Stahlberg G, Jochim A, Haslinger B. Impaired white matter integrity between premotor cortex and basal ganglia in writer's cramp. Brain Behav. 2018;8(10):e01111.
[19] Sondergaard RE, Rockel CP, Cortese F, Jasaui Y, Pringsheim TM, Sarna JR, et al. Microstructural Abnormalities of the Dentatorubrothalamic Tract in Cervical Dystonia. Movement disorders : official journal of the Movement Disorder Society. 2021;36(9):2192-8.
[20] Bonilha L, de Vries PM, Vincent DJ, Rorden C, Morgan PS, Hurd MW, et al. Structural white matter abnormalities in patients with idiopathic dystonia. Movement disorders : official journal of the Movement Disorder Society. 2007;22(8):1110-6.
[21] Kim JH, Kim DW, Kim JB, Suh SI, Koh SB. Thalamic involvement in paroxysmal kinesigenic dyskinesia: a combined structural and diffusion tensor MRI analysis. Hum Brain Mapp. 2015;36(4):1429-41.
[22] Pinheiro GL, Guimaraes RP, Piovesana LG, Campos BM, Campos LS, Azevedo PC, et al. White Matter Microstructure in Idiopathic Craniocervical Dystonia. Tremor and other hyperkinetic movements (New York, NY). 2015;5.
[23] Horovitz SG, Ford A, Najee-Ullah MA, Ostuni JL, Hallett M. Anatomical correlates of blepharospasm. Transl Neurodegener. 2012;1(1):12.
[24] Kirke DN, Battistella G, Kumar V, Rubien-Thomas E, Choy M, Rumbach A, et al. Neural correlates of dystonic tremor: a multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging Behav. 2017;11(1):166-75.
[25] Ramdhani RA, Kumar V, Velickovic M, Frucht SJ, Tagliati M, Simonyan K. What's special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Movement disorders : official journal of the Movement Disorder Society. 2014;29(9):1141-50.
[26] Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, et al. Focal white matter changes in spasmodic dysphonia: A combined diffusion tensor imaging and neuropathological study. Brain. 2008;131(2):447-59.
[27] Guo Y, Peng K, Ou Z, Zhong L, Wang Y, Xie C, et al. Structural Brain Changes in Blepharospasm: A Cortical Thickness and Diffusion Tensor Imaging Study. Frontiers in Neuroscience. 2020;14:543802.
[28] Tomic A, Agosta F, Sarasso E, Svetel M, Kresojevic N, Fontana A, et al. Brain Structural Changes in Focal Dystonia-What About Task Specificity? A Multimodal MRI Study. Movement Disorders. 2020.
[29] Guo Y, Peng K, Liu Y, Zhong L, Dang C, Yan Z, et al. Topological Alterations in White Matter Structural Networks in Blepharospasm. Movement disorders : official journal of the Movement Disorder Society. 2021.
[30] Long Z, Xu Q, Miao HH, Yu Y, Ding MP, Chen H, et al. Thalamocortical dysconnectivity in paroxysmal kinesigenic dyskinesia: Combining functional magnetic resonance imaging and diffusion tensor imaging. Movement disorders : official journal of the Movement Disorder Society. 2017;32(4):592-600.
[31] Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(31):9740-7.
[32] Fujita K, Sako W, Vo A, Bressman SB, Eidelberg D. Disruption of network for visual perception of natural motion in primary dystonia. Hum Brain Mapp. 2018;39(3):1163-74.
[33] Vo A, Sako W, Niethammer M, Carbon M, Bressman SB, Ulug AM, et al. Thalamocortical Connectivity Correlates with Phenotypic Variability in Dystonia. Cereb Cortex. 2015;25(9):3086-94.
[34] Li L, Lei D, Suo X, Li X, Yang C, Yang T, et al. Brain structural connectome in relation to PRRT2 mutations in paroxysmal kinesigenic dyskinesia. Human Brain Mapping. 2020;41(14):3855-66.
[35] Sako W, Fujita K, Vo A, Rucker JC, Rizzo JR, Niethammer M, et al. The visual perception of natural motion: abnormal task-related neural activity in DYT1 dystonia. Brain. 2015;138(Pt 12):3598-609.
[36] Cheng FB, Wan XH, Feng JC, Ma LY, Hou B, Feng F, et al. Subcellular distribution of THAP1 and alterations in the microstructure of brain white matter in DYT6 dystonia. Parkinsonism & related disorders. 2012;18(8):978-82.
[37] Yang J, Luo C, Song W, Guo X, Zhao B, Chen X, et al. Diffusion tensor imaging in blepharospasm and blepharospasm-oromandibular dystonia. J Neurol. 2014;261(7):1413-24.
[38] van der Meer JN, Beukers RJ, van der Salm S, Caan MW, Tijssen MA, Nederveen AJ. White matter abnormalities in gene-positive myoclonus-dystonia. Movement Disorders. 2012;27(13):1666-72.
[39] Vo A, Eidelberg D, Ulug AM. White matter changes in primary dystonia determined by 2D distribution analysis of diffusion tensor images. Journal of magnetic resonance imaging : JMRI. 2013;37(1):59-66.
[40] Berman BD, Honce JM, Shelton E, Sillau SH, Nagae LM. Isolated focal dystonia phenotypes are associated with distinct patterns of altered microstructure. NeuroImage Clinical. 2018;19:805-12.
Figures
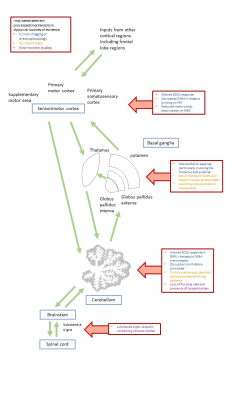


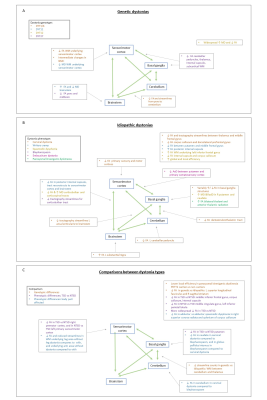
Figure 3: Clinical characteristics and results of studies. Sorted by dystonia type. ROI= region of interest; MC= manifesting carrier; NMC= non-manifesting carrier; WM= white matter; FA= fractional anisotropy; RadD= radial diffusivity; AxD= axial diffusivity; MD= mean diffusivity; (L)= left; (R)= right; BSM= blepharospasm; OMD= oromandibular dystonia; CD= cervical dystonia; HC= healthy control; Add= adductor form; Abd= abductor form