2677
Sub-Normothermic Ex-Vivo Perfusion of Porcine Kidney Grafts Improves Energy Metabolism: 31P-MRSI Analysis1Department of Radiology and Medical Informatics, University of Geneva, Geneva, Switzerland, 2Department of Vascular Surgery, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland, 3Center for Biomedical Imaging (CIBM), Geneva, Switzerland, 4Department of Surgery, Geneva University Hospitals and Medical School, Geneva, Switzerland
Synopsis
Improved preservation strategies for the storage of graft collected after circulatory death could increase the number of kidneys available and improve patient survival. Warm (22 and 37°C) ex-vivo perfusion has emerged as a feasible strategy for organ recovery, but the underlying mechanism remains elusive. Using phosphorus magnetic resonance spectroscopic imaging (31P-MRSI) and histological scoring, we evaluated kidney viability and adenosine triphosphate (ATP) production during sub-normothermic ex-vivo kidney perfusion (SNOP) versus hypothermic machine perfusion (HMP) in a porcine kidney autotransplantation model.
Introduction
Transplantation is the preferred treatment for end-stage kidney disease, but it suffers from a severe shortage of available organs. This shortage has led to the expansion of the donor pool beyond standard-criteria kidney donors, including extended criteria donors (ECD) and donated after circulatory death donors (DCD)1,2. Magnetic resonance imaging (MRI) is a well-established clinical diagnostic tool for assessing kidney graft function3. In addition to proton imaging, MRI enables detection of high-energy phosphate metabolites with 31P magnetic resonance spectroscopic imaging (31P-MRSI). Using 31P-MRSI, we seek to demonstrate that sub-normothermia (22°C) perfusion of porcine kidney grafts promote ATP production and graft viability by reducing ischemic-reperfusion injury (IRI).Methods
Porcine kidneys underwent 60 minutes of warm ischemia with cross-clamping of the renal arteries, before being harvested to mimic donation after cardiac arrest. Kidneys were randomly assigned to the following groups: Hypothermia at 4°C with (100kPa pO2, HMOP) or without oxygen (HMP) or Sub-normothermia at 22°C with oxygen (SNOP). They were perfused ex-vivo using a homemade MRI-compatible “pulsatile perfusion machine” as previously published4.During ex-vivo perfusion, energy metabolites were assessed using 31P-MRSI. Measurements were performed on a Pisma-fit 3T whole body multi-nuclear MRI scanner (Siemens). 1H imaging was acquired using embedded body coil. 31P-MRSI was performed using a single 31P loop coil fixed at the bottom of the perfusion tank4. Phosphorus sequence consisted of 3D spatial encoding, with a matrix size of 16x16x8, for a nominal spatial resolution of 15.6x15.6x20 mm3. Elliptical encoding with 18 weighted averages resulted in an acquisition time of 45 min. The inorganic phosphate (Pi) resonance (5.2ppm), was used as a reference for the chemical shift signal. The metabolites (α-, β-, and γ- ATP, PME, Pi, PCr) were fitted with Gaussian peaks using the syngo software (SIEMENS) and were estimated over all the kidneys by averaging voxels containing kidney tissue. The concentration of Pi in the buffer (25 mM)4 was used as reference to estimate the concentration of ATP and PME.
Cortical kidney biopsies were obtained at baseline, after warm ischemia, at the end of the ex-vivo perfusion and 1h after transplantation. Histopathological analysis score was performed based on those described by Goujon et al.5,6 using Zen software (Zeiss). The following 6 categories were assessed: Glomerular integrity, tubular dilatation, brush border integrity, cellular debris in lumina of tubules, interstitial edema, and tubular cell vacuolization. Each score was converted to a final scale from 0 to 5 for a maximum cumulative score of 30. 30 was the highest score corresponding to the more severe damage.
Results
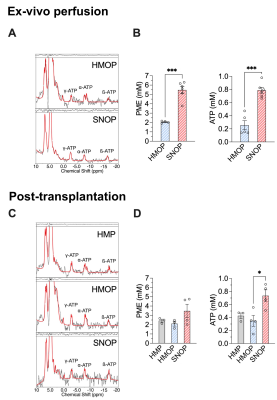
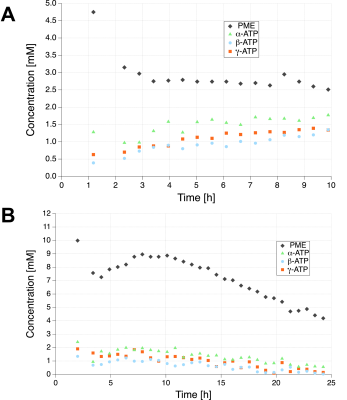
Figure 1 shows 31P-MRSI spectra with concentration of PME and ATP for the three groups before and after transplantation. Kidney ATP levels are reduced by warm ischemia and correlate with pretransplant renal injury4. During ex-vivo perfusion at 22°C, kidney graft PME and ATP levels were markedly higher than in grafts perfused at 4°C with oxygen (5.5 vs 2.1mM and 0.79 vs 0.26mM).Figure 2 displays time courses of PME and ATP levels in the SNOP kidneys before and after transplantation. PME concentration was 4 times higher than ATP at the beginning of the perfusion (4mM) and rapidly decreased until it reached a plateau (2mM) and remained stable over time. After 10 hours of perfusion at 22°C, ATP levels gradually decreased to an undetected value after 42 hours.
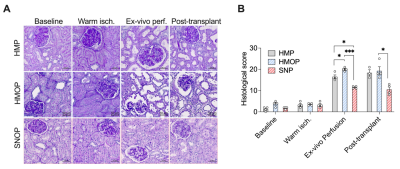
Figure 3 shows cortical kidney biopsy and histological Goujon score. No significant damage was observed after warm ischemia. Visible damage on HMOP and HMP histologies corresponds to significantly higher Goujon score at the end of the perfusion and after reperfusion. Sub-normothermic perfusion leads to the greatest protection from IRI during transplantation (score after SNOP, HMOP and HMP: 12.5, 20, and 16.5).
Discussion
We found that perfusion of kidneys at 22°C with oxygenated solution increased renal ATP production and protected against IRI during transplantation, compared with hypothermic perfusions.ATP concentrations in the kidney increased gradually up to 10 hours of perfusion. After 10 hours of perfusion at 22°C, ATP levels gradually decrease to 0 at 42 hours of perfusion. This is consistent with our hypothesis that at 22°C, kidneys are metabolically active9,10. Interestingly, at 4°C, in the absence of warm ischemia, ATP levels remained stable up to 22 hours of perfusion, but at lower levels (0.26mM)4.
The assessment of the kidney viability measured with ATP is in agreement with our histo-pathological findings as sub-normothermic showed the least cellular damage in comparison to other preservation conditions. Our results are consistent with previous literature showing that ex-vivo normothermic kidney perfusion significantly reduced preservation injury and improved function up to 8 days post transplantation7 and that perfusion of the DCD kidney with blood at 22 °C for 4 hours resulted in a significant reduction of kidney acute tubular necrosis and apoptosis when compared to 15 °C and normothermia (37 °C)8.
Conclusion
In kidneys, SNOP improved graft viability when compared with hypothermic perfusions. These results suggest that SNOP might mitigate the negative effect of warm ischemia as shown by the histological scoring and promote renal metabolism, including ATP production. In addition, 31P-MRSI provides a non-invasive tool to assess kidney viability prior to transplantation. Altogether, these improvements should reduce the number of discarded kidneys and improve patient survival.Acknowledgements
This study was supported by the Swiss National Foundation to Jean-Marc Corpataux, François Lazeyras (SNF 320030_182658) and Alban Longchamp (SNSF PZ00P3-185927). As well as the Mendez National Institute of Transplantation, and the Leenards Foundation to Alban Longchamp and by the Center for Biomedical imaging (CIBM) of the Universities of Geneva and Lausanne, the University Hospitals of Geneva and Lausanne and the EPFL.
References
1. Assis-Borba L, Cristelli MP, Paula MI, Franco MF, Tedesco-Silva H, Medina-Pestana JO. Expanding the use of expanded criteria donors in kidney transplantation. Int Urol Nephrol. Aug 2014;46(8):1663-71.
2. Summers DM, Watson CJ, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int. Aug 2015;88(2):241-9.
3. Wang YT, Li YC, Yin LL, Pu H, Chen JY. Functional assessment of transplanted kidneys with magnetic resonance imaging. World J Radiol. Oct 28 2015;7(10):343-9.
4. Longchamp A, Klauser A, Songeon J, et al. Ex Vivo Analysis of Kidney Graft Viability Using 31P Magnetic Resonance Imaging Spectroscopy. Transplantation. Sep 2020;104(9):1825-1831.
5. Meier RPH, Piller V, Hagen ME, et al. Intra-Abdominal Cooling System Limits Ischemia-Reperfusion Injury During Robot-Assisted Renal Transplantation. Am J Transplant. Jan 2018;18(1):53-62.
6. Goujon JM, Hauet T, Menet E, Levillain P, Babin P, Carretier M. Histological evaluation of proximal tubule cell injury in isolated perfused pig kidneys exposed to cold ischemia. J Surg Res. Apr 1999;82(2):228-33.
7. Mazilescu LI, Urbanellis P, Kaths MJ, et al. Prolonged Normothermic Ex Vivo Kidney Perfusion Is Superior to Cold Nonoxygenated and Oxygenated Machine Perfusion for the Preservation of DCD Porcine Kidney Grafts. Transplant Direct. Oct 2021;7(10):e751.
8. Bhattacharjee RN, Ruthirakanthan A, Sun Q, et al. Subnormothermic Oxygenated Perfusion Optimally Preserves Donor Kidneys. Kidney Int Rep. Sep 2019;4(9):1323-1333.
9. Buchs JB, Lazeyras F, Bühler L, et al. [The viability of kidneys tested by gadolinium-perfusion MRI during ex vivo perfusion]. Prog Urol. May 2009;19(5):307-12.
10. Lazeyras F, Buhler L, Vallee JP, et al. Detection of ATP by "in line" 31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs' kidneys. MAGMA. Oct 2012;25(5):391-9.
Figures