2676
Compartment-Specific Metabolic Investigation of 3D Cell Culture by Real-time NMR for Investigating Metabolic Diseases
Christian Urzi1,2,3, Damian Hertig1,2, Christoph Meyer1,2,3, Jean-Marc Nuoffer2,4, and Peter Vermathen1
1Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Bern, Switzerland, 2Institute of Clinical Chemistry, University Hospital Bern, Bern, Switzerland, 3Graduate School for Cellular and Biomedical Sciences, University of Bern, Bern, Switzerland, 4Department of Pediatric Endocrinology, Diabetology and Metabolism, University Children’s Hospital of Bern, Bern, Switzerland
1Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Bern, Switzerland, 2Institute of Clinical Chemistry, University Hospital Bern, Bern, Switzerland, 3Graduate School for Cellular and Biomedical Sciences, University of Bern, Bern, Switzerland, 4Department of Pediatric Endocrinology, Diabetology and Metabolism, University Children’s Hospital of Bern, Bern, Switzerland
Synopsis
In this study was shown the possibility to perform compartment-specific metabolic investigations of living fibroblasts in real-time using a bioreactor system within an NMR spectrometer. Cell volume and the ratio cells to extracellular medium within the sensitive region of NMR coil were evaluated for quantification purposes. The needed sensitivity to detect metabolite changes in the extracellular footprint at different flow rates was shown. The capability of diffusion technique to distinguish between compartment-specific contributions was revealed, and to detect changes in compartment-specific metabolite pools under standard or selective culture conditions, and upon inhibitor challenges.
Background
Mitochondria are the cell’s powerhouse and important signalling organelles [1]. Mitochondria dysfunction leads to a large group of disorders that often appear as a consequence of defects in the oxidative phosphorylation (OXPHOS) but altered mitochondrial signalling affecting cellular metabolism may be even more important. Therefore the simultaneous monitoring of metabolism and bioenergetic function may provide new insight into mitopathogenic mechanisms [2]. We have previously shown the feasibility to measure in real-time mitochondrial respiration and metabolic information of 3D matrix embedded fibroblasts by using a perfused NMR bioreactor system [3]. However, absolute metabolite quantification was not possible, because the exact cell volume was unknown, and intracellular metabolism was not studied directly, since detected NMR spectra included both contributions from cells and medium. Therefore, the capability to separately measure the metabolic intracellular fingerprints and extracellular footprints would allow a better characterization of ongoing bioenergetic and metabolic processes. For this reason, we first aim at determination of cell volume and of the ratio cells to extracellular medium within the sensitive region of the NMR tube for metabolite quantification. Secondly, we aim at compartment-specific metabolic investigations utilizing diffusion differences between compartments in order to separate intra- and extracellular metabolites. Thirdly, we aim at extracellular metabolic footprint characterization by measuring the outflowing supernatant from the perfused bioreactor.Methods
A perfused NMR bioreactor for investigating living cells embedded in a collagen-based scaffold (Cultrex™), using the InsightCellTM flow tube with a standard 5mm NMR probe head has previously been established by our group [3] [Figure 1]. NMR experiments were performed on a 500MHz Bruker Avance II spectrometer. Online metabolic profiling by proton NMR was performed using 1D PROJECT pulse sequence with water presaturation and a T2-filter of 80 ms [4], a data size of 32 K points, 64 transients, an acquisition time of 2.73 s, and a relaxation delay of 4 s (≈8min per spectrum). Diffusion measurements (16 steps with increasing b-values, Δ = 74.4 ms and δ = 6 ms) were performed for investigating intra- and extracellular metabolic signal intensities separately [6]. Cell viability was controlled by detection of LDH release and trypan blue staining. Mitochondrial stress tests were performed as feasibility tests to monitor Glycolytic-, Glutamine-, and other pathways. Scaffold volume and shrinking were evaluated by light microscopy imaging and 23Na NMR, respectively. Percentage cell volume within the tube was determined by 23Na NMR making use of sodium concentration differences between intra- and extracellular space [5], then validated by diffusion behaviour of water. Cell footprints were determined via spectral subtraction of the pure cell medium spectrum from the out-flowing supernatant spectra.Results and Discussion
The implemented perfused bioreactor system within the NMR spectrometer allows detection of perfusion rate dependent metabolite content. We determined dynamic profiles of 35 metabolites and measured interleaved diffusion behaviour of 19 metabolites and water [Figure 2, Figure 4]. The results revealed a scaffold volume reduction over time which depended on the number of cells embedded (5, 10, and 20 million fibroblasts), with a decrease from 37% to 74% after cell encapsulation over a period of 24 h. Cell volumes of 10 and 20 million fibroblasts were found to occupy 1.4% and 2.9%, respectively of the total sample volume. Diffusion measurements of supplemented DMSO and Mannitol in standard perfused condition of fibroblasts were performed to validate the capability of diffusion technique to separate compartment-specific contributions [Figure 3]. As expected, DMSO showed a bi-exponential decay, being permeable to the cell membrane. Mannitol, instead, showed only a fast mono-exponential decay, being localized only in the extracellular space. We observed a biexponential decay for all observed 19 metabolites and water, confirming their distribution in both compartments [Figure 4] and allowing thus a separation of intra- and extracellular contributions. Water diffusion led to a compartmentalized water of 1 % of the total water volume in the NMR sample, in accordance with the percentage cell volume. Different perfusion conditions showed a dependence of intra- and extracellular metabolite pools on perfused culture media. Addition of complex I inhibitor rotenone led to an upregulation of glycolysis and to a cellular metabolic adaptation on complex II-dependent respiration [Figure 5]. Extracellular footprint was determined by collecting supernatants from the bioreactor outlet at different flow rates (0.2, 0.1, 0.05 ml min-1), providing additional information on the metabolic ongoing processes. Clear metabolic differences were observed depending on the applied flow-rate, demonstrating the sensitivity of the method to monitor metabolic cell status. Independently from the order of applied flow rates, we observed an upregulation of glycolysis and glutaminolysis at lowering the flow rate.Conclusion
This study shows the possibility to perform compartment-specific metabolic investigations of living fibroblasts in real-time using a bioreactor system within an NMR spectrometer. A 3D cell culture sample characterization was performed by evaluating scaffold volume and shrinkage, and by determining the cell volume and the ratio cells to extracellular medium within the sensitive region of NMR coil. The sensitivity to detect metabolite changes in the extracellular footprint at different flow rates was shown. The capability is demonstrated to distinguish between compartment-specific metabolic contributions by diffusion techniques, and to detect changes in both intra- and extracellular metabolite pools under standard condition, and upon inhibitor challenges.Acknowledgements
Supported by the Swiss National Science Foundation (SNSF #310030_192691).References
- Chan, C. David. Mitochondria: Dynamic Organelles in Disease, Aging, and Development. Cell 2006; 125 (7), 1241-1252.
- Smeitink J. A. M. Mitochondrial disorders: Clinical presentation and diagnostic dilemmas. Journal of Inherited Metabolic Disease 2003; 26 (2-3), 199-207.
- Hertig D, Maddah S, Memedovski R, Kurth S, Moreno A, Pennestri M, Felser A, Nuoffer JM, Vermathen P. Analyst. 2021;146(13):4326-4339.
- Aguilar J, Nilsson M, Bodenhausen G, Morris G. Spin echo NMR spectra without J modulation. Chemical Communications 2012; 48(6), 811-813.
- Mancuso A, Fernandez E, Blanch H, Clark D. A nuclear Magnetic Resonance technique for determining hybridoma cell concentration in hollow fiber bioreactors. Biotechnology 1990; 8, 1282–1285.
- Van Zijl P, Moonen C, Faustino P, Pekar J, Kaplan O, Cohen J. Complete separation of intracellular and extracellular information in NMR spectra of perfused cells by diffusion-weighted spectroscopy. Proceedings of the National Academy of Sciences of the United States of America 1991; 88(8), 3228-3232.
Figures
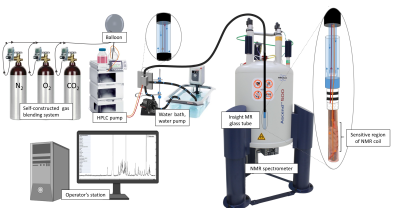
Setup of the cell culture bioreactor system inside the NMR
spectrometer.
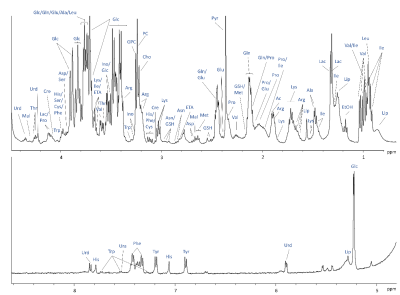
1H NMR spectrum of Cultrex embedded FBCO04 with assignments of selected
metabolites identified.
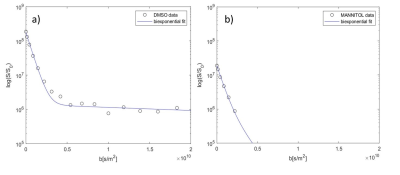
Substance spiking
experiments. A: 1 mM DMSO was spiked in the inlet and the diffusion behaviour
characterized. As expected, DMSO shows a
bi-exponential decay (extrac: 97.7±1.0%; intrac: 2.3±1.0%), being permeable to
the cell membrane. B: 0.25 mM of cell membrane impermeable Mannitol was spiked
in the inlet and the diffusion behaviour characterized. As expected, Mannitol
shows only a fast monoexponential decay, being localized only in the
extracellular space.
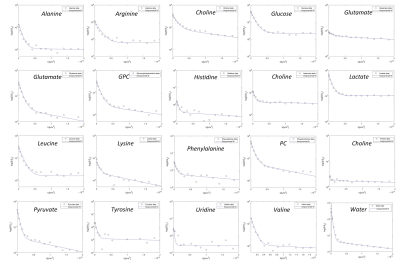
Metabolites and water signal intensity as a function of
diffusion-weighting factor b. All observed 19 metabolites and water show
a bi-exponential decay, being
distributed in both intra- and extracellular compartments.
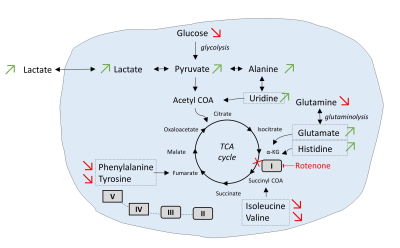
Changes of intracellular metabolites pool upon addition of
inhibitor of complex I rotenone. Addition of the inhibitor led to an
upregulation of glycolysis and to a cellular metabolic adaptation on complex
II-dependent respiration. Green arrows: metabolites which concentration
increases upon rotenone addition; Red arrows: metabolites which concentration
decrease upon rotenone addition.
DOI: https://doi.org/10.58530/2022/2676